Saad Mohamed1*, Ahmad El-Askary2 and Ahmed Abd-Eltawab3
1
Department of Pediatrics, Faculty of Medicine, Al-Azhar University (New Damietta), Egypta
2 Department of Medical Biochemistry, Faculty of Medicine, Al-Azhar University (New Damietta), Egypt
3
Department of Physiology, Faculty of Medicine, Beni-Suef University, Egypt
*Corresponding Author: Saad Mohamed, Department of Pediatrics, Faculty of Medicine, Al-Azhar University (New Damietta), Egypt.
Received: September 10, 2018; Published: October 24, 2018
Citation: Saad Mohamed., et al. “Environmental Tobacco Smoke Exposure and Relation to Asthma Exacerbations and Severity among Primary School Children”. Acta Scientific Paediatrics 1.4 (2018): 20-26.
Background: Asthma is a common childhood disease that is greatly affected with environmental triggers. Passive smoking, environmental tobacco smoke (ETS), could exacerbate asthma symptoms and affects control of asthma and subsequently children' quality of life. Parents frequently underreport their children's exposure to environmental smoke. Cotinine levels could be a reflection of passive exposure to the cigarette smoke.
Objective: The aim of the present study is to assess the magnitude of environmental tobacco smoke exposure among Egyptian asthmatic children, and to elucidate the effect of measured versus reported smoke exposure on asthma severity and asthma exacerbations.
Patients and Methods: A cross sectional study included 350 asthmatic primary school children (6 - 12 years) recruited from Pediatrics and Pulmonology clinics at Al-Azhar University Hospital (New Damietta), during the period from January 2016 to July 2017. The diagnosis of asthma was based on typical asthma symptoms. Asthma severity was determined according to GINA guidelines. A complete history, clinical examinations were performed. Serum cotinine was detected by ELISA. Patients were classified according to serum cotinine level into: (1) no smoke exposure (cotinine < 0.05 ng/mL); (2) positive smoke exposure (cotinine ≥ 0.05 ng/mL).
Results: The mean age of studied children was 8.24 ± 1.73 years. There was discrepancy between reported smoke exposure by parents (47.4%) and environmental smoke exposure as detected by serum cotinine (66.3%). Environmental smoke exposure was associated with high frequency of moderate and severe persistent asthma (P: < 0.001). Cotinine level was significantly associated with disturbance of sleep (P: 0.021), wheezing during exercises (P: 0.005), oral steroid prescription (P: 0.009) and use of controller medications (P: < 0.001). There was poor association between self-reported exposure and asthma exacerbations. Exposure to ETS determined by serum cotinine levels > 0.05 ng/ml was positively associated with asthma exacerbations. Although not always statistically significant, associations for asthma exacerbations were always present.
Conclusion: Environmental tobacco smoke was frequent among Egyptian asthmatic children. ETS exposure was significantly associated with asthma severity and exacerbations. Serum cotinine is a better indicator of ETS exposure than reports obtained from parents; thus, serum cotinine should be considered as an indicator for ETS exposure for better risk stratification of childhood asthma.
Keywords: Cotinine; Asthma; Passive Smoking; Child; Environmental; Tobacco; Secondhand Smoke
Asthma is the leading chronic illness of childhood and an increasingly prevalent disease that overly affects low-income children [1]. Asthma is a chronic disease of the respiratory system causing recurrent cough, shortness of breath, and wheezing [2]. The symptoms result from airway obstruction and may resolve spontaneously or as a result of treatment [3].
Asthma pathogenesis is complex and interactions between genetic, epigenetic, and environmental factors predispose patients to develop a number of dysfunctional immunologic regulatory patterns [4]. Acute asthma exacerbations are characterized by airway constriction and increased mucous production that in turn lead to symptoms of cough, wheeze, shortness of breath, and chest tightness [5].
There exist a number of external factors with a potential to influence the development and course of allergic diseases [6].
Environmental tobacco smoke (ETS), a mixture of gases and particles from the burning cigarette and exhaled main stream smoke, contains more than 1014 oxidative molecules per puff of smoke, including both nitric oxide and superoxide [7].
Worldwide, there are almost 1 billion male and 250 million female smokers. Global Youth Tobacco Survey showed that almost half of the world's children are exposed to second-hand tobacco smoke (SHS) [8]. Such exposure to smoke may be particularly dangerous for children suffering from bronchial asthma as it amplifies the effect of other airway irritants, increases the incidence of exacerbation, and aggravates the disease course [9].
As a result of exposure to tobacco smoke, epithelial cells of bronchial passages get damaged and an inflammatory process follows. Smoking attracts neutrophilis to, and increases their number in, the lungs [10].
The best way to identify ETS exposure is unclear [11]. Classifying smoking status by self-report alone may be unreliable because children may have ETS exposure outside their own homes or because caregivers may under-report household smoking [12,13].
Cotinine is a nicotine biomarker measurable in the blood, urine or saliva. Cotinine levels increase with greater exposure to ETS [14]. Household smoking is associated with higher cotinine levels among asthmatic children [15,16]. Many children considered nonexposed to tobacco smoke have elevated cotinine levels. Parental surveys about sources of ETS do not adequately predict children’s cotinine levels [17].
The relationship between passive smoking and asthma morbidity in children is also well recognized [18]; however, the current literature related to asthma severity and passive smoking has elicited conflicting results. Some studies have linked passive smoking to increased asthma prevalence, poorer asthma control, and, increased symptoms [19].
In Egypt, information is lacking as regard to the prevalence and effects of passive smoking especially on asthmatic children. Thus, the aim of this work is to study the magnitude of environmental tobacco smoke exposure among Egyptian asthmatic children, and to elucidate the link between smoke exposure with asthma control and severity.
Across sectional study included350asthmatic primary school children (6-12 years) recruited from Pediatrics and Pulmonology clinics at Al-Azhar University Hospital (New Damietta), during the period from January 2016 to July 2017.
The diagnosis of asthma was based on typical asthma symptoms such as recurrent wheeze, cough and breathlessness resolving spontaneously or with an inhaled bronchodilator. Asthma severity was determined according to GINA guidelines [20].
Exclusion criteria included patients with upper or lower respiratory tract infections, any genetic or hereditary lung diseases such as cysticfibrosis or bronchiectasis, known cardiovascular disease, a neuromuscular disorder, musculoskeletal deformities or a restrictive pulmonary defect.
After obtaining informed consent, Patients’ demographic characteristics were recorded. A complete history, clinical examinations, serum cotinine and spirometry were also performed.
Asthma exacerbations were detected using a set of outcomes about wheezing asked during history taking [21].
A sample of serum was collected from all attendants in the study after confirmation of diagnosis and determination of severity of asthma. The samples were carried on ice and then transferred to the-80ºC fridge until cotinine measurement. Serum cotinine were measured using an ELISA kit(according to manufacturer protocols).Based on the recommendations for serum cotinine cut points to distinguish smoke exposure from non-smoke exposure of AvilaTang., et al. [22] and Benowitz., et al. [23], the following categories were used:
Spirometry was done using (MEDISOFT–HYPERAIR compact + flow meter pulmonary function testing-Belgium). Spirometricindices were calculated using the best out of three technically satisfactory performances in accordance to the recommendations of the American Thoracic Society (ATS) [24].
The study was approved by the local ethical committee. Written informed consent was obtained from parents.
Data were analyzed using the SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). Quantitative data were expressed as the mean ± standard deviation (SD). Chi square test was used to compare variable. Risk detection was done using binary logistic regression analysis. For all tests, significance was considered if p < 0.05.
The mean age of studied children was 8.24 ± 1.73 years. Frequency of males (55.2%) and rural residents (67.5%) were higher than females and urban residents. There was discrepancy between reported smoke exposure by parents (47.4%) and environmental smoke exposure as detected by serum cotinine (66.3%) as shown in table 1.
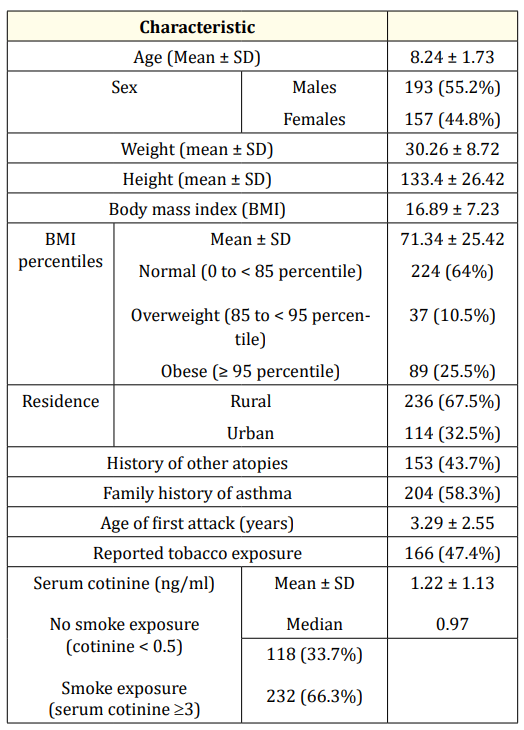
Table 1: General characteristics of studied cases.
Patients were classified based on Spirometry according to GINA classifications. Environmental smoke exposure was associated with high frequency of moderate and severe persistent asthma (P: <0.001). In addition, FEV1 was significantly lower among non-exposed group as shown in table 2.
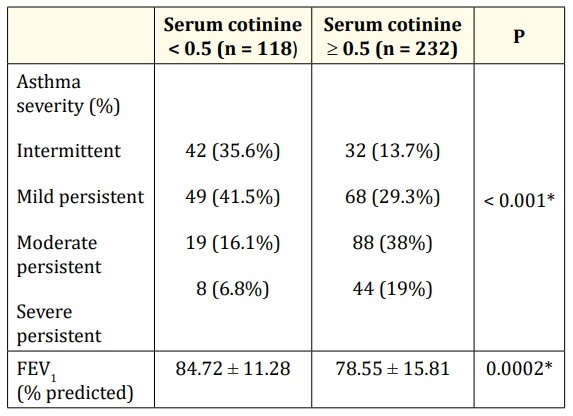
Table 2: Asthma severity in relation to passive smoking. *: significant
Environmental smoke exposure was significantly associated with disturbance of sleep (P: 0.021), wheezing during exercises (P: 0.005), oral steroid prescription (P: 0.009) and use of controller medications (P: < 0.001). There was no association between smoke exposures with school abstinence, health care or emergency room visits as shown in table 3.
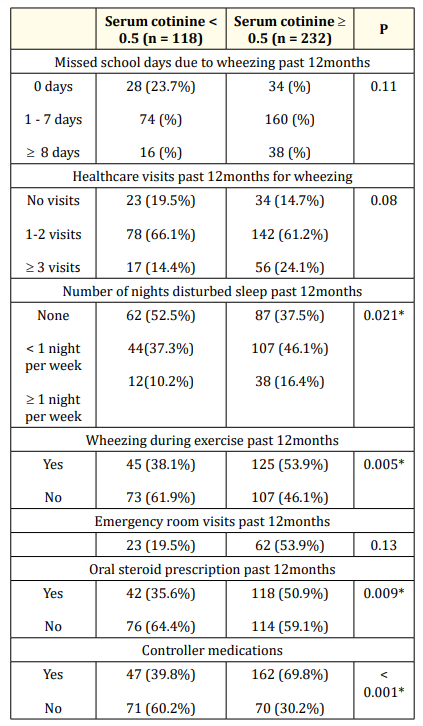
Table 3: Asthma exacerbations in relation to passive smoking. *: significant
Table 4 demonstrates the associations between dualistic variables of self-reported and cotinine-based (cut-off of 0.05 ng/mL) smoke exposure and asthma outcomes. Associations between selfreport of any current smoker in the household and asthma exacerbation variables were not strong, but self-reported exposures were associated with increased odds of asthma severity reaching statistical significance (OR: 1.79, 95% CI 1.28to 2.50). Exposure to any ETS determined by serum cotinine levels > 0.05 ng/ml was positively associated with all asthma outcomes. Although not always statistically significant, associations for asthma exacerbations were always present.
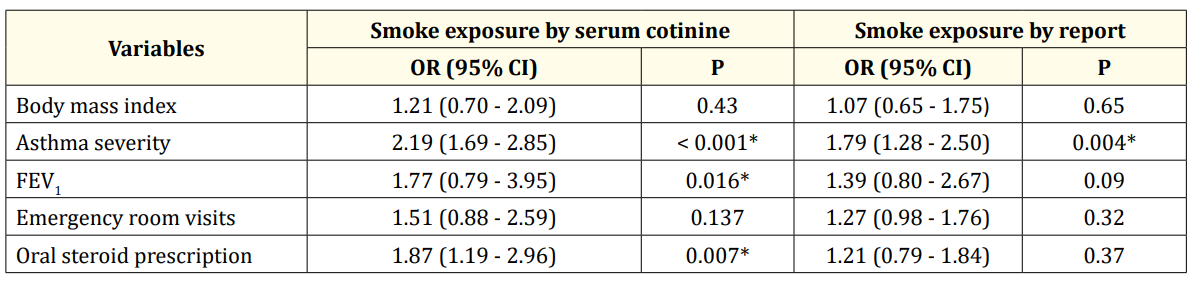
Table 4: logistic regression analysis of smoke exposure by report versus by cotinine level. *: significant
Environmental tobacco smoke exposure causes many health consequences, and specifically in subjects with asthma this exposure is associated with a more severe disease course and a higher frequency of exacerbations [7,25].
ETS exposure may be a combination of second-hand smoke and third-hand smoke, generally defined as exposure to tobacco smoke that previously settled onto furniture, clothes and inanimate objects and subsequently becomes re-suspended [26-29].
Serum cotinine, a specific and major metabolite of nicotine with a half-life of approximately20 hours, has been established as a valid marker of recent exposure [30].
Associations between asthma outcomes in children and ETS exposure have been reported in the literature with exposures associated with increased risk of asthma, asthma exacerbations, wheezing and reduced lung function [31,32]. Tobacco smoke is a mixture of compounds, including carbon and nitrogen oxides, particulate matter, nitrosamines, polycyclic aromatic hydrocarbons, carbonyls and other chemicals, many of which are known toxicants that can induce inflammation and altered immune responses [33].
The present study confirms that parental reports are not always reliable when used to screen for ETS exposure.
Discrepancies between reported smoking and biological measures of tobacco exposure may be due to under-reporting of reported household smoking in the research setting due to social desirability bias or to recall bias [14]. Alternatively, children may have tobacco exposures outside of the home that are unrecognized by caregivers, such as through incursions of smoke from other units in multiunit housing [34] or exposures in non-household social settings [35]. Findings of the present work confirm previous results that suggest the importance of valid marker of smoking exposure for accurate classification of risk factors associated with severe asthma outcomes.
There is a limited number of studies on asthma-related outcomes that have directly compared the effects of cotinine to those of reported smoking have had mixed findings. Two studies demonstrated that both maternal reports of smoking and cotinine levels are associated with asthma exacerbations, lung function, and incidence of asthma or asthma-like symptoms [36,37]. However, other researchers found that maternal serum cotinine was a better predictor of early life wheezing than parent-reported exposure [38] and salivary cotinine was a better predictor of lung function than passive ETS exposure determined by questionnaire [39].
Biochemical assessment of ETS exposure allows for more objective characterization of tobacco smoke exposure and epidemiologic assessment of exposure-outcome relationships unburdened by exposure misclassification error seen in self-reported measures of exposure. Serum cotinine, the biomarker used to determine exposure in the current study, is the major proximate metabolite of nicotine and has been considered a reliable, objective measure of SHS exposure for some time [40]. Nicotine and its metabolites, however, are not necessarily causative agents of the adverse effects of tobacco smoke examined in this study, with effects most likely driven by other compounds.
In summary, the present study demonstrated the increased odds of asthma exacerbations and poor asthma control with environmental smoke exposure as detected by serum cotinine than by parents' reports, representing the importance of adding objective measure for accurate assessment of an important asthma trigger.
Environmental tobacco smoke was intensely frequent among Egyptian asthmatic children. ETS exposure was significantly associated with asthma severity and exacerbations, with subsequent affection of children's quality of life and consumption of health resources. Serum cotinine is a better indicator of ETS exposure than reports obtained from parents; thus, serum cotinine should be considered as an indicator for ETS exposure for better risk stratification of childhood asthma.
None declared
Copyright: © 2018 Saad Mohamed., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.