Elizabeth KE1*, Ashwin David Ashok2, Sobhakumar S3 and Sujatha TL4
1Former Professor and Head, Department of Pediatrics, SAT Hospital, Government Medical College, Thiruvananthapuram, Kerala, India
2Research Associate, SAT Hospital, Government Medical College, Thiruvananthapuram, Kerala, India
3Professor of Pediatrics, SAT Hospital, Government Medical College, Thiruvananthapuram, Kerala, India
4Additional Professor of Obstetrics and Gynecology, SAT Hospital, Government Medical College, Thiruvananthapuram, Kerala, India
*Corresponding Author: Elizabeth KE, Former Professor and Head, Department of Pediatrics, SAT Hospital, Government Medical College, Thiruvananthapuram, Kerala, India.
Received: September 04, 2018; Published: September 20, 2018
Citation: Elizabeth KE., et al. “Large and Small-for-Gestational-Age (LGA and SGA) Babies born to Mothers with Pre-Pregnancy/Gestational Diabetes Mellitus (PPDM/GDM) Vs. No-DM””. Acta Scientific Paediatrics 1.3 (2018): 23-28.
Objective: Assess outcome of small and large-for gestational-age (SGA and LGA) babies born to a cohort of mothers with pre-pregnancy and gestational diabetes mellitus (PPDM and GDM) and No-DM.
Design: Cohort Study.
Setting: Tertiary-care hospital.
Patients: Cohort of 410 mothers enrolled before 6 weeks of gestation, categorized into PPDM GDM and No-DM subgroups and their newborn babies.
Intervention: Nutrition, Metformin and or Insulin and Protocol based neonatal care.
Main Outcome Measures: Optimum/suboptimum glycemic control, Neonatal weight, gestational age, morbidity, mortality and NICU stay.
Main Outcome Measures: PPDM was 19.5%, including 70 mothers already diagnosed as DM, GDM 39% and No-DM 41.5%. Detection rate of PPDM was 5.6% and GDM was 17.5%. Majority with PPDM and GDM required insulin and two-third had optimum glycemic control. Appropriate-for-Gestational-Age more with good glycemic control, SGA: 54%, 26%, 21%, LGA: 9.6%, 5.9%, 0.5% respectively. Significant parameters in PPDM Vs. GDM: SGA (RR 2.1, 95% CI 2.9 - 3.6), Congenital anomalies (RR 3.3, 95% CI 5.1 - 8.8), Neonatal mortality (RR 4, 95% CI 2.1 - 3.2), Prematurity and NICU admission with longer stay. Macrosomia and birth injury were more in GDM. Hypoglycemia longer stay in NICU and macrosomia were more with poor glycemic control.
Conclusions: A change in profile with more SGA and less LGA babies noted. This can modify short-term and long-term including transgenerational outcome, like early onset of DM in future. Differential short-term outcome noted, based on onset of DM and glycemic control. Pre-pregnancy/early first trimester screen followed by second and third trimester screen and optimum glycemic control, throughout pregnancy, recommended.
Keywords: Pre-Pregnancy Diabetes Mellitus (PPDM); Gestational Diabetes Mellitus (GDM); Appropriate for Gestational Age (AGA) Babies; Small for Gestational Age (SGA) Babies; Large for Gestational Age (LGA) Babies; Macrosomia
The prevalence of Non-Communicable Diseases (NCDs), especially Diabetes Mellitus (DM) is increasing globally. Screening for DM in pregnancy is undertaken after 16 - 24 weeks. Pre-pregnancy and early first trimester screen are not routinely done in most developing countries and hence, some mothers with pre-pregnancy DM (PPDM) may get labelled as gestational DM (GDM). This may lead to a missed opportunity of maintaining euglycemia during the early period of organogenesis resulting in diabetic embryopathy and congenital anomalies [1,2]. Even though large-for-gestational-age (LGA) babies are expected, many small-for-gestational-age (SGA) babies are born to mothers with DM. It is known that neonatal outcome varies with respect to PPDM and GDM [3], in comparison to mothers with No-DM. Hence, a study was undertaken to compare the neonatal parameters like SGA, LGA and outcome among babies born to mothers with PPDM, GDM and No-DM.
A cohort of 410 mothers, enrolled before 6 weeks of gestation in a tertiary care Government hospital, were followed up till delivery. 70 mothers with DM before pregnancy and 23 mothers, who had HbA1C > 6.5% in early first trimester, confirmed as per ADA, 2011 criteria [4] formed the PPDM subgroup. 187 mothers, who were screen negative at enrolment and later confirmed as GDM by one-step-75g OGTT as per IADPSG, 2010 criteria [5] at 24 - 28 or 32 - 34 weeks' gestation formed the GDM group. 200 mothers, who remained screen negative throughout pregnancy formed the NoDM group. All with DM were initiated on dietary therapy as per ADA, 2008 criteria [6]. Metformin and insulin were added as per ADA, 2011 guidelines [4]. Maternal medications and glycemic control were recorded. Mean glucose levels, fasting < 110 and 2 hours post-prandial < 140 mg/dl were considered as optimum control, as per DIPSI, 2013 [7]. Plasma glucose was measured by enzymatic hexokinase method (Cobas 6000, Roche Diagnostics) and HbA1c by HPLC method in NABL accredited lab, attached to the institution. Neonatal parameters like birth weight, gestational age, multiple pregnancy, congenital anomalies, metabolic derangements, indication and duration of NICU care and mortality were recorded and compared. Neonatal diagnosis and management, including criteria for NICU admission were based on National Neonatology Forum (NNF), India, 2011 guidelines [8]. Research Committee and Institutional Ethics Committee approval and informed consent from participants were obtained prior to study. Data were analyzed using SPSS version 16.0 for Windows. Descriptive statistics was used for participant characteristics and Fisher's exact/Chi square tests for proportions.
Out of total 480 pregnancies, there were 481 babies. There were three neonatal mortalities, two in PPDM and one each in the other subgroups and one intrauterine death in GDM subgroup. The age of mothers ranged from 19 - 37 years and the mean age was comparable in three subgroups (P > 0.05). Majority of them belonged to middle or low socio-economic status. Maternal overweight and obesity were more in PPDM and GDM subgroups. The PPDM group consisted of 93 mothers and 94 babies, including a pair of twins (19.5%). 187 mothers and 187 babies with one pair of twins; excluding one intrauterine death, formed GDM group (39%) and 200 mothers and their babies formed No-DM group (41.5%). The baseline characteristics in the various subgroups are summarized in table 1. The proportion of mothers with DM, detected at early first trimester screen before six weeks with evidence of hyperglycemia, starting at least three months prior to diagnosis, that included pre-pregnancy period, was 5.6% (PPDM subgroup) and those with second and third trimester screens was 17.5% (GDM subgroup). 76 mothers (81.7%) with PPDM and 118 (63.1%) with GDM were on insulin and 60 (64.52%) in PPDM and 127 (67.91%) in GDM group had optimum glycemic control. Caesarian Section rate was 42.55%, 36.9% and 34% and NICU admission was 90.42%, 62.03% and 22% respectively in PPDM, GDM and No-DM subgroups.
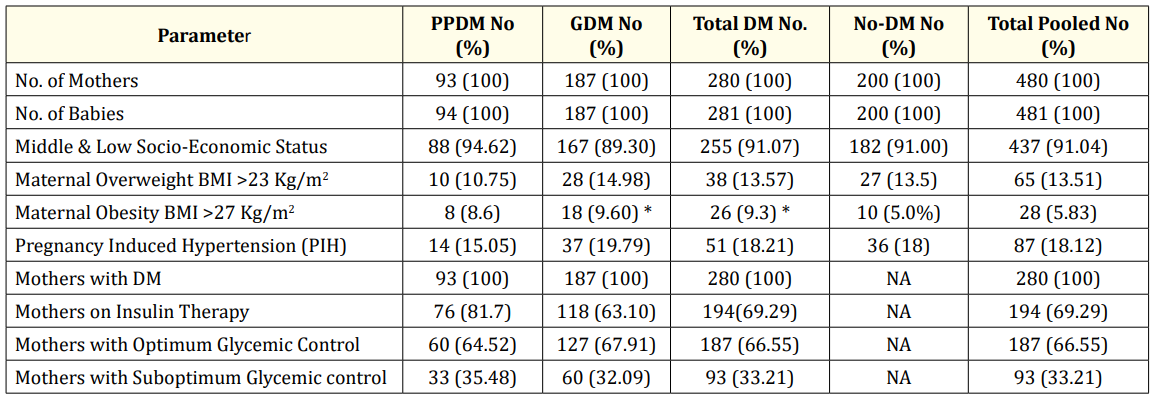
Table 1: Baseline Characteristics ans Socio-demographic Profile in the various Subgroups: Pre-Pregnancy DM (PPDM),
Gestational DM (GDM), Total DM, No-DM and Total Pooled.
* Significant P value < 0.05.
Neonatal parameters and outcome showed significant differences in the three subgroups (Table 2). Differential outcome was noted with respect to birth weight, gestational age, NICU admission and duration of stay, hypoglycemia, hypocalcemia, meconium stained amniotic fluid, birth trauma and shoulder dystocia. Hyperbilirubinemia and sepsis were comparable in all three subgroups. Adverse outcome and morbidity were more in those with DM compared to No-DM. Appropriate- for-Gestational-Age (AGA) babies were 34.04%, 63.1% and 78%, respectively in PPDM, GDM and NoDM subgroups. AGA babies were more in those with optimum glycemic control. SGA babies were 54%, 26 % and 21 % and LGA babies were 9.6%, 5.9% and 0.5% respectively. Neonatal issues were less in No-DM subgroup; Congenital anomalies were more in DM than No-DM (RR 5, CI 9.9 - 12.8). The following parameters were significant in PPDM vs. GDM; SGA babies (RR 2.1, 95% CI 2.9 - 3.6), Congenital anomalies (RR 3.3, 95% CI 5.1 - 8.8), Neonatal mortality (RR 4, 95% CI 2.1 - 3.2), NICU admission with longer stay, and prematurity (P < 0.05). Macrosomia > 4.5 Kg in term babies and birth trauma like brachial plexopathy and fracture clavicle were more in GDM subgroup (P < 0.05). Hypoglycemia, NICU admission with longer stay and macrosomia were more in those with poor glycemic control (P < 0.05). Duration of NICU stay was more in PPDM; 16 +/- 0.6 compared to 8 +/- 0.5 in GDM and 1 +/- 0.5 days in No-DM.
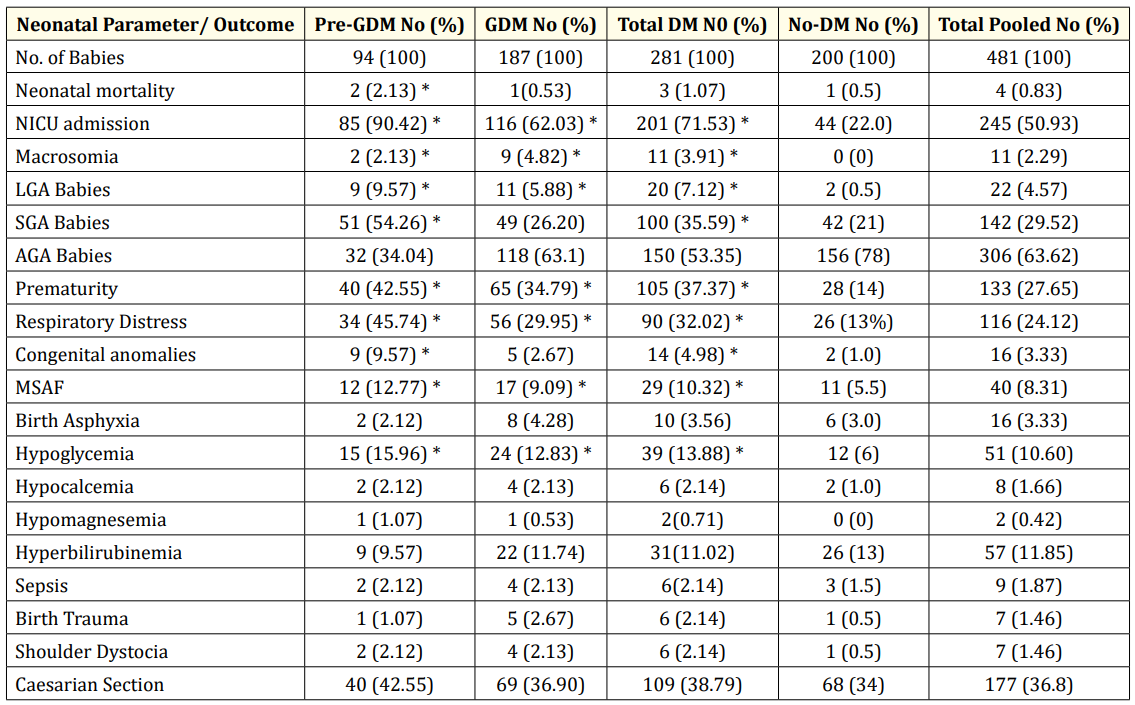
Table 2: Clinical Profile and Neonatal Outcome in the various Subgroups: Pre-Pregnancy DM (PPDM), Gestational DM (GDM), Total DM,
No-DM and Total Pooled Subgroups.
* Significant P value < 0.05.
Among those with congenital anomalies, cardiac defects like shunt lesions, d-TGA, TOF and Asymmetric septal hypertrophy (ASH) were noted in two-third of babies and non-cardiac defects like ano-rectal malformation and ectrodactyly in the rest. There was one baby with Down syndrome, AV canal defect and anal atresia in PPDM subgroup.
In the present study, the proportion of mothers with DM, detected at early first trimester screen before six weeks of gestation was 5.6%. They were included as PPDM, due to raised HbA1C in the last three to four months, which comprised of pre-pregnancy and early pregnancy period. This is of great concern as screening for GDM is routinely done around 20 weeks or later, as pregnancy related hyperglycemia is rare before this period. But, this can lead to a missed opportunity of ensuring glycemic control, at least in some mothers, during the phase of organogenesis. This can result in embryopathy, congenital anomalies and SGA babies. Hence, HbA1C screen, followed by a one-step 75g OGTT is recommended [9] at registration, starting from early first trimester or pre-pregnancy visit. The use of HbA1c is highlighted, in view of the previous report of inadequacy of plasma glucose in the diagnosis of DM, especially in Asian women [10]. This approach is relevant due to the current epidemic proportions of DM and Non-Communicable Diseases (NCDs) among the general population. The proportion of mothers with GDM has been reported to be 2.4-24% [11,12] and 17% in one study from the same region [13], comparable to 17.5%, noted in the present study.
In the present study, glycemic control was estimated as mean glucose levels; fasting < 110 and 2 hr. post-prandial < 140 mg/dl [7], as against the latest revised criteria of fasting glucose < 95 and 2 hr. post-prandial < 120 mg/dl, that came in after the initiation of the study in 2017 [14]. Majority in PPDM and GDM were on insulin and more than two-third had optimum glycemic control. Achieving glycemic control during pregnancy is essential for better feto-maternal outcome [15]. Medical and nutritional care in DM complicating pregnancy has been reviewed and standardized by different working groups [15,16]. CS rate of around 40% noted in the present study among mothers with DM, was comparable with No-DM subgroup and the recent Auckland study [17]. Some previous studies have reported higher CS rates up to 74% [18].
Neonatal complications were more in PPDM and GDM subgroups and outcome varied with respect to the onset of DM and glycemic control. Neonatal anthropometric measurements showed a changing profile compared to other studies with respect to LGA and SGA babies [19,20]. This may be attributable to the differences in dietary pattern, lesser weight gain during pregnancy and low pre-pregnancy BMI among the participants. High LGA and macrosomia rate up to 28% has been reported from a North India in 2011 [21]. Insulin therapy is reported to reduce macrosomia [22]. Optimum control resulted in more AGA babies in the present study. Hypoglycemia, NICU admission with longer stay and macrosomia were more in those with poor glycemic control. Proportion of macrosomia and birth trauma were significantly more in GDM subgroup. The proportion of SGA babies was as high as 54% in PPDM subgroup, as against 24%, reported from the North Indian study [21]. This may be attributable to diabetic embryopathy and restrictive dietary intake among the participants. Intra-uterine growth retardation (IUGR) resulting in SGA babies has poor immediate outcome and early onset of adulthood diseases like DM, as per Barker Hypothesis [23]. LGA and Macrosomia results in more immediate complications and future obesity. NICU admission with longer stay, SGA, prematurity and congenital anomalies were more in PPDM subgroup. These findings are in accordance with other reported studies [16,24]. Proportion of prematurity was three-fold more in PPDM than No-DM; which was on par with the Auckland study [17]. Proportion of prematurity is liable to vary as per the decision for elective deliveries and CS. Respiratory distress was noted in nearly half in PPDM and GDM, in comparison to 10% reported from the North Indian study [21]. Metabolic complications like hypoglycemia, hypocalcemia, hyperbilirubinemia was less compared to other studies [18,21]. Macrosomia and birth trauma like brachial plexopathy and fracture clavicle were more in the GDM group [21,25]. The proportion of congenital anomalies was around 10%, on par with the North Indian study [21]. Among congenital anomalies, cardiac defects were noted in two-third; shunt lesions, d-TGA, TOF and asymmetric septal hypertrophy (ASH), which is a fore runner of hypertrophic obstructive cardiomyopathy (HOCM). HOCM is reported as one of the causes of unexplained sudden mortality in these babies. A five-fold increase in cardiac defects has been reported in mothers with DM [26]. The non-cardiac defects were anorectal malformation and ectrodactyly. Caudal regression syndrome, which is a known anomaly [27]. was not noted in the present study. Mortality in the present study was low compared to other previous studies [28, 29], which is attributable to the protocol based neonatal care [8]. The clinical profile and neonatal outcome were different in PPDM and GDM, compared to No-DM subgroup, as observed in other studies [28,30]. This warrants early pre-pregnancy or first trimester screening and optimum glycemic control throughout pregnancy.
A change in profile with more SGA and less LGA babies were noted. This is of public health importance, in view of short-term as well as long-term and transgenerational outcome, including early onset of DM in the offspring. A differential neonatal outcome was noted with respect to onset of DM and glycemic control, among mothers. Hence, pre-pregnancy/early first trimester screen with HbA1C, followed by one-step- 75 mg OGTT, along with second and third trimester screen and optimum glycemic control, throughout pregnancy is recommended.
The help of the Obstetrics and Neonatology team and the financial support from the Kerala State Board of Medical Research are gratefully acknowledged. Preliminary data from this study was presented as poster in the International Congress of International Pediatrics Association (IPA) at Vancouver, Canada, August 2016 (IPA 2016-435).
EKE conceived the study, was the clinical consultant and drafted the paper, ADA contributed in data management, analysis and drafting. SSK was the neonatology consultant and STL was the Obstetric consultant.
Nil.
Copyright: © 2018 Elizabeth KE., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.