ELMeneza S*, Almorsy E and Mahmoud
Pediatric Department (NICU), Faculty of Medicine for Girls Al-Azhar University, Cairo, Egypt
*Corresponding Author: ELMeneza S, Pediatric Department (NICU), Faculty of Medicine for Girls Al-Azhar University, Cairo, Egypt.
Received: August 07, 2018; Published: September 07, 2018
Citation: Sangita D Kamath., et al. “A Case Report on Choroidal Metastases from Adenocarcinoma of Lung – Tip of the Iceberg!”. Acta Scientific Paediatrics 1.3 (2018):00-00.
Background: Birth asphyxia is an important cause of static developmental and neurological handicap both in term and preterm infants, despite the increasing understanding of the mechanism leading to perinatal asphyxia, early determination of brain damage following hypoxic-ischemic events still remains one of the hardest problems in neonatal care. EEG is an excellent tool to help in diagnosing non-clinical seizures or to avoid a misdiagnosis of seizures in the presence of atypical non-ictal neonatal behavior. When used selectively through serial recordings, neonatal EEG greatest value is its potential for prediction of short and long-term prognosis.
Aims: The aim of this study was early detection of non-clinical seizures in neonates with perinatal asphyxia utilizing EEG monitor and to assess the short term outcome.
Patients and Method: 50 full term neonates with a history of perinatal events suggestive of asphyxia were considered in this study. Their gestational age ranged from 37 to 42 weeks. Neonates with multiple congenital anomalies were excluded. All cases were subjected to full history taking, physical examination including estimation of gestational age and Apgar score, and laboratory investigations including cord ABG, CBC, electrolytes, Kidney function, liver function and blood glucose. The studied cases were divided into two groups according to presence or absence of clinical manifestation of seizure. All cases were monitored with EEG, and each group was subdivided based on the EEG discharges.
Results: No significant difference found between the studied groups regarding demographic, clinical, or laboratory data (p > 0.05). 22% of the cases had clinical seizures while 78% had no clinical seizures. All of the cases with clinical seizures showed ictal EEG discharges (electroclinical seizure), while 51% of the cases with no clinical seizures showed ictal EEG discharges (electrographic seizure). The most common EEG abnormalities were spikes and sharp waves. Three cases with electrographic seizers (15% of the electrographic seizures) died, while all other infants were discharged to home.
Conclusion: It can be concluded that monitoring brain function of full term infants with HIE can detect brain seizure activity and help starting appropriate management and avoid further brain injury.
Recommendations: Trials of early introduction of AEDs in cases with HIE and ictal EEG discharge in the 1st 24 hours of life. Further studies should be conducted on the long-term prognosis of asphyxiated babies having ictal EEG discharges without apparent clinical seizures.
Keywords: EEG Monitoring; Non-Clinical Seizures; Newborn Infants; Perinatal Asphyxia
Birth asphyxia is an important cause of static developmental and neurological handicap in both full-term and preterm infants (3% to 13% with cerebral palsy has evidence of intrapartum asphyxia). Despite the increasing understanding of the mechanism leading to and resulting from perinatal asphyxia, early determination of brain damage following hypoxic-ischemic events remains one of the hardest problems in neonatal care [1].
EEG interpretation have to be correlated to the gestational age as well as postnatal age during the neonatal period. It can severe also as tool for determination of gestational age [2].
Neonatal EEG provides useful information that reflects the function of neonatal brain. EEG may assist in determining brain maturation and identify focal or generalized abnormalities, existence of potentially epileptogenic foci or ongoing seizures. It is useful in assessing prognosis for neonates at risk for neurological sequelae.
EEG is an adjuvant to the clinical determination of seizures. Because of immaturity of the C.N.S., ictal and interictal abnormalities can take many, often nonspecific, forms.
EL Meneza and Mostafa studied the relation between cerebral blood flow (CBF) and the electroencephalographic (EEG) activity during the acute insult and reperfusion state of perinatal asphyxia (PA). They found that transient dysmaturity pattern of EEG was correlated to lowered CBF mean velocity (Mv) of ACA at the 12 hrs of age, while infants with permanent dysmature EEG pattern had increase in the mean values of the ACA and MCA during the first forty-eight hours of life. As persistence dysmaturity is correlated with long term poor outcome in high-risk neonates, they studied the role of the brain growth factors, PDGF beta as a contributing factor to dysmaturity [3].
Neonates commonly have abnormal movements of uncertain meaning. It is often difficult to determine whether the movements represent seizures or not. Routine EEG may be inadequate to electrographically-confirm suspected seizures. In difficult cases, several studies or continuous recordings with computer scored methods are more reliable.
The aims of this study were early detection of seizures in fullterm neonates having hypoxic-ischemic encephalopathy (HIE) that have no clinical manifestation utilizing EEG monitor, and the assessment of short term outcomes.
This cohort study was carried out at Al Zahra University Hospital, after an informed written consent obtained from mothers of all infants before getting them involved in the study, the cases were selected randomly.
The study included 50 full-term neonates with perinatal asphyxia. Their gestational age ranged from 37 to 42 weeks, 26 (52%) were males while 24 (48%) were females. Neonates suffering from perinatal asphyxia and having one or more of the following (according to AAP and AGOG, 2006) [4] are included in this study: presence of intrapartum fetal distress signs, Apgar score < 5 at five minutes, Profound metabolic or mixed acidemia on an umbilical cord arterial blood sample, clinical neurological sequelae in the immediate neonatal period (HIE) and evidence of multi organ system dysfunction. Preterm neonates postdate neonates, and neonates with multiple congenital anomalies were not included.
All cases were subjected to full history taking, physical examination including Apgar score, gestational age estimation using new Ballard score [5]. Severity of encephalopathy was detected by using the sarnat clinical stages of HIE. Laboratory investigations included Cord blood gases, CBC, kidney and liver function, blood glucose and electrolytes.
All neonates under this study were monitored with EEG and observed for appearance of clinical seizures. Based on the clinical observation, the studied cases were divided into two groups: (1) cases with clinical seizure, (2) cases without clinical manifestation of seizure. The first group included 11 cases, while the second group included 39 cases.
Nihon Kohden, BSM-8301 Life Scope 9.
Electroencephalography (EEG) monitor was used in this study. It has 4 channel electrodes, which were connected to scalp of neonates using a conductive paste, as shown in figure 1. Recording of EEG activity was done for at least 60 minutes. Good preparation of infant scalp was done by avoidance of caput succedaneum which may impede conduction and shaving of hair over the sites of connection of electrodes. Assessment of state of sleep was necessary to differentiate between EEG discharges during sleep and wake states.
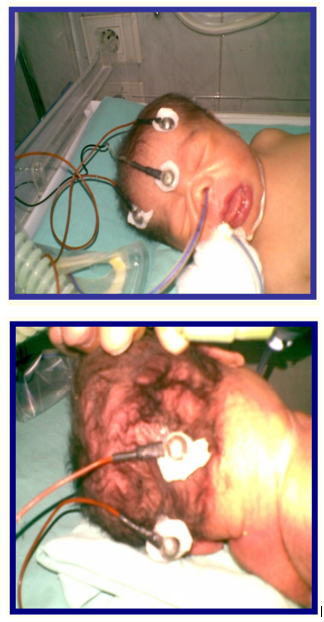
Figure 1: EEG electrodes connected to the infant’s head.
Artifacts (as moving, crying, suckling) were identified during the EEG recording to avoid misdiagnosis. In some cases, diagnosis was confirmed by conventional EEG. The diagnostic criteria for electrical seizure activity in continuous EEG were rhythmic, repetitive, stereotypic waveforms with an evolution of morphologic features, amplitude, or electric field, lasting more than 10 seconds.
Based on the EEG discharges, further division for each group of the cases was performed, as shown in figure 2.
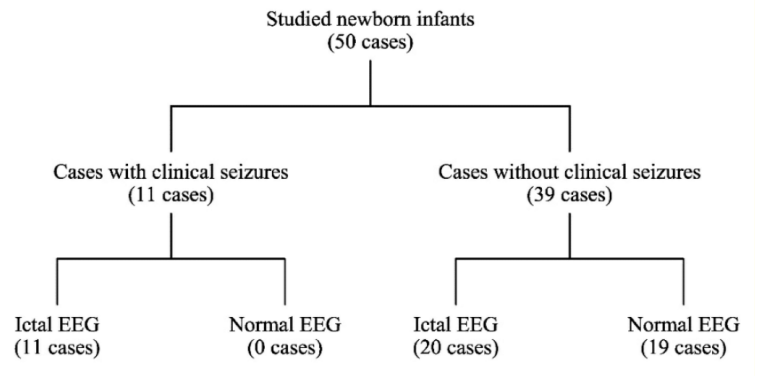
Figure 2: Division of the study cases.
The statistical analysis was performed using MS Excel version 10. Both student's (t-test) and Chi-square (χ2 ) test were utilized in the statistical analysis of the collected information.
Table 1 summarizes the demographic data of the studied cases. There was no statistical significance among the demographic data between infants having normal and abnormal EEG discharges (p > 0.05).
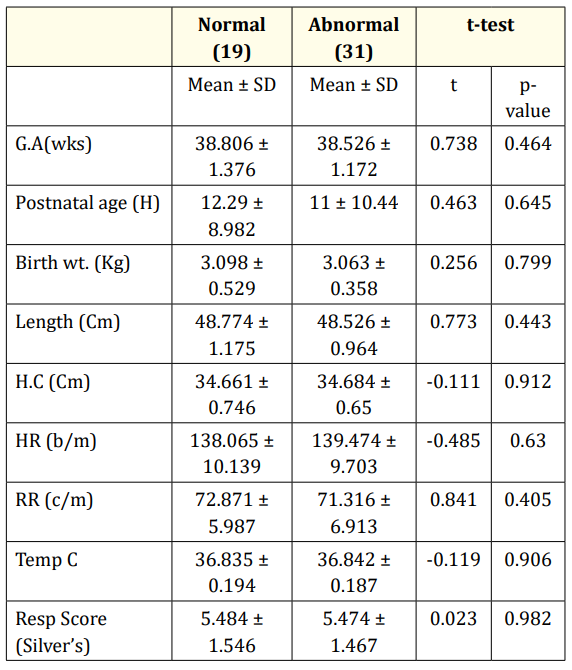
Table 1: Clinical and Demographic data description of the studied newborn infants at the time of admission.
Table 2 summarizes the cord blood gases of the studies cases. There was no statistical significance among the blood gases data between infants having normal and abnormal EEG discharges (p > 0.05).
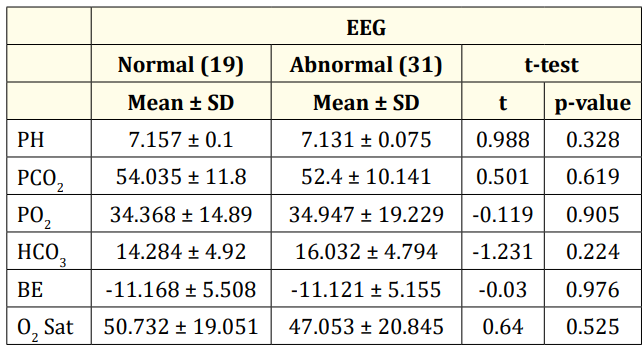
Table 2: Cord blood gases among the studied group.
Table 3 summarizes the results of the laboratory investigations of the studies cases. There was no statistical significance among the laboratory investigation data between infants having normal and abnormal EEG discharges (p > 0.05).
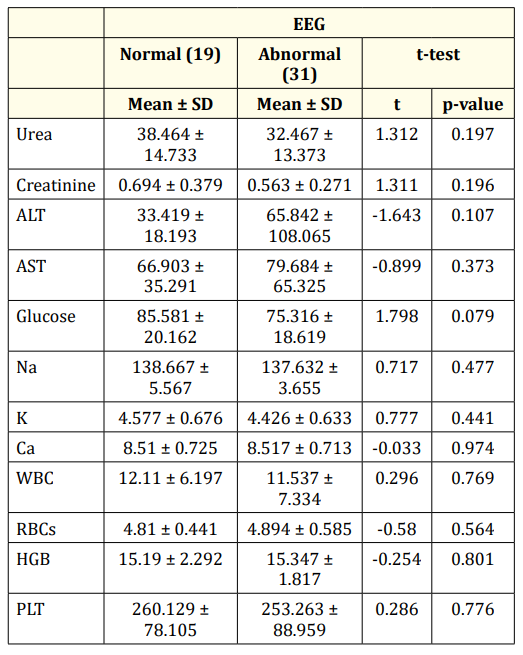
Table 3: Laboratory investigations of the studied groups.
Tables 4 and 5 summarize resuscitation and risk factors of the studied cases, respectively. 22% of the cases had clinical seizures while 78% had no clinical seizures (Figure 3). All of the cases with clinical seizures showed ictal EEG discharges (electroclinical seizure), while 51% of the cases with no clinical seizures showed ictal EEG discharges (electrographic seizure) (Figure 4)
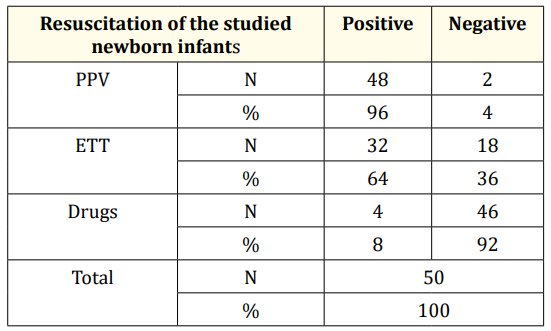
Table 4: Resuscitation of the studied newborn infants.
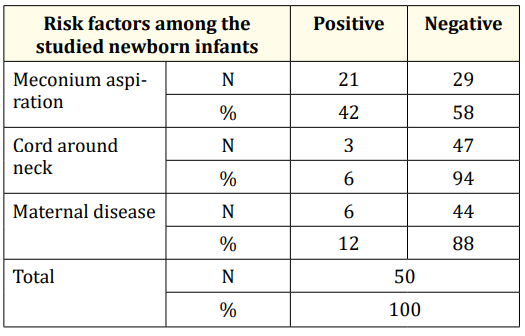
Table 5: Risk factors among the studied groups.
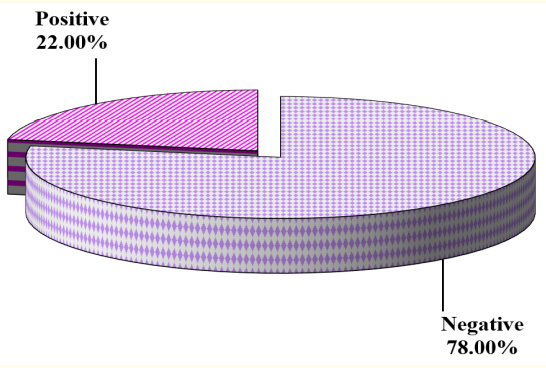
Figure 3: Cases with clinical seizures.
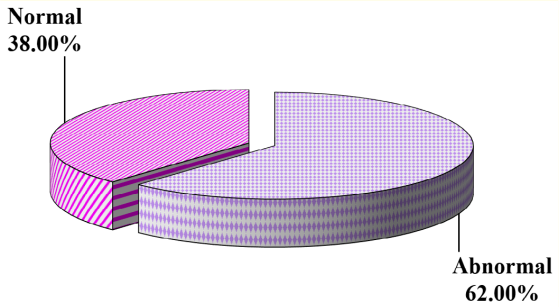
Figure 4: EEG finding in the studied groups.
Most of the abnormal EEG discharges are characterized by spikes and sharp waves, as shown in table 6.
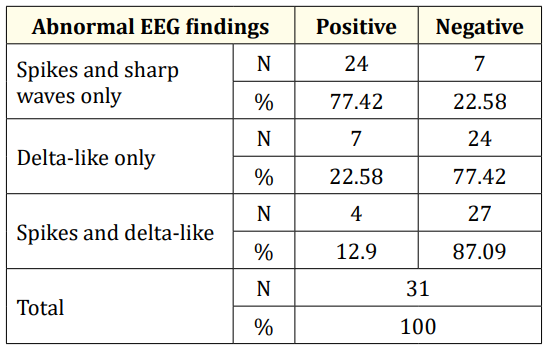
Table 6: Abnormal EEG findings in the studied groups.
Figure 5: Abnormal EEG discharges as observed
Perinatal asphyxia is the third leading cause of neonatal death and the main cause of long-term neurodevelopment handicap throughout the world. It is described as a dramatic phenomenon in newborns by altering cerebral blood flow regulatory mechanisms and causing loss of vascular auto-regulation, tissue ischemia and reperfusion leading to cell death and tissue damage [6].
Seizures are an early sign of brain injury in newborns. These seizures are in most cases repetitive or associated with asymptomatic electrographic seizures. Despite the relative resistance of the immature brain to seizure-induced brain damage, there is evidence that neonatal seizures impair normal brain development [7].
Electrographically documented neonatal seizures with or without clinical manifestations represent the most accurate and current concept of neonatal seizures [8].
In our study, me conium aspiration was the most common risk factor for asphyxia (21cases, 42%). This is in agreement with Shresha., et al. 2009 they studied the risk factors of HIE and show that meconium staining amniotic fluid was the commonest risk factor reaching up to 65% of cases [9]. Also Synder and Cloherty 2008 reported meconium aspiration as risk factor for asphyxia due to the resultant failure of adaptation of the neonatal cardiopulmonary circulation to stress [10].
Although most neonatal EEG patterns are non-specific and cannot provide the diagnosis when used alone, it is conversely true that they should cue the clinician to order neuroimaging tests. EEG is also an excellent tool to help diagnose subclinical seizures or to avoid a misdiagnosis of seizures in the presence of atypical nonictal neonatal behaviors [11]. Concerning EEG finding in our study there were 19 cases (38%) with normal EEG and 31 cases (62%) with ictal activity.
21 cases (53%) had ictal activity only without appearance of clinical seizure. This in agreement with Divyen., et al. (2008) who reported that about 63% of cases had ictal activity without clinical correlation to electrical activity on the study of accuracy of EEG monitoring for seizure detection in term infants [12].
Non-clinical seizures are not uncommon in critically ill patients in NICU and were recorded in 34% of patients undergoing continuous EEG in NICU and in 37% of comatose patients without signs of seizure activity. In the Columbia study seizures were detected in 19% of patients who had continuous EEG monitoring. The seizures were exclusively non-clinical seizures in 92% of patients.
Our results are in agreement with Murray., et al. 2008 who reported that only one-third of neonatal EEG seizures displays clinical signs on simultaneous video recordings [13]. Also they reported that two-thirds of these clinical manifestations are unrecognized or misinterpreted by experienced neonatal staff. They confirm that in the recognition and management of neonatal seizures clinical diagnosis alone is not enough.
Among the 11 cases suffering from clinical seizures (HIE II) in our study, 7cases (63%) had ictal activity before the onset of clinical seizures. This agreed with Murthy (2004). He said that presence of EEG ictal episodes is demonstrating the presence of ongoing seizure activity on the study of value of continuous EEG monitoring in evaluation of non-convulsive seizures [14].
Spikes and sharp waves are associated with the same clinical correlation a statistically significant increased risk for recurrent seizures. Spikes and sharp waves share morphological features; the sharp contour and paroxysmal occurrence that are identified as epileptiform activity. Spikes and sharp waves may occur in focal, multifocal, or bilaterally synchronous distribution with the accompanying implications of electrophysiological dysfunction in similar focal, multifocal, or bilaterally synchronous pattern.
There was significant increase in the mean cerebral blood flow velocity in anterior cerebral artery (ACA) and middle cerebral artery(MCA) during ictal activity, the mean percentage change in velocity ranged from 12.5% to 50%. The increase in CBFV was significantly higher with tonic seizures than clonic or subtle. The increase of CBF was associated with spikes, theta alpha beta frequency, paroxysmal and burst suppression background [16].
Van Rooij., et al. 2010 confirmed that seizures are common in full-term infants with HIE and a substantial portion of neonatal seizures are subclinical. Immediate treatment of both clinical and subclinical seizures reduced the total duration of seizure patterns, which suggests a possible reduction of brain injury.
Continuous EEG monitoring allows the detection of seizures often when there are no clinical indications of such activity [17]. This makes continuous EEG an excellent method for supplementing single or serial recordings in the detection and management of non-clinical seizures. This finding was also confirmed by the work of [14].
Concerning the outcome of our studied neonates, (15%) of cases with sub-clinical seizures died. This was in contrast to the finding of Peliowski and Finer (1992) with approximately 62% of their studied newborns with HIE died [18].
McBride., et al. 2000 reported that there are association between the amount of electrographic seizure activity and subsequent mortality and morbidity in at-risk infants in general and in infants with perinatal asphyxia. Only with more effective treatment of neonatal electrographic seizures can their potential contribution to poor neurodevelopmental outcome, independent of degree of insult, be ascertained [19].
On the contrary to our results Bye., et al. 1995 found that the occurrence of ictal seizures was not associated with higher mortality and morbidity. They found that EEG background was the only significant predictor of survival at 1 month and that a higher number of independent discharging foci was associated with poor outcome at 1 year [20]. Long term outcome among infants with ictal seizures was studied by McBride., et al. 2000 [19], there was association with an increased risk of severe CP, microcephaly, and failure to thrive irrespective of seizure.
Excessive slow background and excessive interhemispheric voltage were also detected among cases with sever HIE. Moderate abnormal EEG was diagnosed when EEG was immature for conception age or contained at least one of the following patterns; Generalized low voltage activity with normal age findings [21].
Limitation of this study was the limited channel system that necessitate the change the position of the electrodes. Also the connections of electronic equipment and artifacts from electronic devices and non-electronic equipment. Electrode placement issues and biological including movement-related artifacts or during nursing care.
We can conclude that monitoring brain function. of full term infants with HIE is important to detect brain seizure activity and help to start appropriate management and avoid further brain injury.
Trials of early introduction of AEDs in cases with ictal activity in the 1st 24 hours of life. Further studies should be conducted on the long term prognosis of asphyxiated babies having ictal EEG discharges without apparent clinical seizures.
Copyright: © 2018 ELMeneza S., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.