Mohamed Shawky Elfarargy1,2*, Elsharkawy HM1, Elgendy MM1, Nassar MA1, Attia GF3, Abu Elmaaty MK4, Mohamed SA5, Abd-Ellatif AA6,7, Alaa A Mohamed8,9 and Kelleni MT10,11
1Pediatric Department, Faculty of Medicine, Tanta University, Egypt
2Pediatric Department, College of Medicine, Jouf University, KSA 3Clinical Pathology Department, Faculty of Medicine, Tanta University, Egypt 4Obestetric and Gynecology Department, Faculty of Medicine, Tanta University, Egypt 5Pediatric Department, Faculty of Medicine (Damietta) – Al-Azhar University, Egypt 6Physiology Department, Faculty of Medicine, Beni-Suef University, Egypt 7Physiology Department, College of Medicine, Jouf University, KSA 8Biochemistry Division, Pathology Department, College of Medicine, Jouf University, KSA 9Department of Biochemistry, Faculty of Medicine, Beni-Suef University, Egypt 10Pharmacology Department, Faculty of Medicine, Minia University, Egypt 11Pharmacology Department, College of Medicine, Jouf University, KSA*Corresponding Author: Mohamed Shawky Elfarargy, Pediatric Department, Faculty of Medicine, Tanta University, Egypt.
Received: July 16, 2018; Published: August 02, 2018
Citation: Mohamed Shawky Elfarargy., et al. “Study of Lactate and Nucleated Red Blood Cells as Early Predictors of Neonatal Hypoxic Ischemic Encephalopathy”. Acta Scientific Paediatrics 1.2 (2018):03-08.
Background: Hypoxic ischemic encephalopathy (HIE) after prenatal asphyxia is an important cause of neonatal morbidity.
Aim: Study nucleated red blood cells per 100 white blood cell (NRBC/100 WBC) counts and lactate levels in cord blood as early markers of neonatal HIE.
Patients and Methods: This is a prospective case control study conducted on 60 HIE neonates, nucleated red blood cells count/100 WBCs and lactate level in the cord blood was measured. These were compared to 60 neonates matched in age, sex and body weight apparently healthy neonates as a control group.
Results: Nucleated red blood cells per 100 white blood cell (NRBC/100 WBC) counts and lactate levels in cord blood were higher in HIE neonates than control group with a significant difference (P value < 0.0001).
Conclusion: NRBC/100 WBC counts and lactate levels in cord blood could be used as an early markers of neonatal HIE.
Keywords: Lactate; Nucleated Red Blood Cells; Hypoxic Ischemic Encephalopathy (HIE)
Hypoxic ischemic encephalopathy (HIE) after prenatal asphyxia is an important cause of neonatal morbidity, neurological disability and mortality. The early prediction of hypoxic ischemic encephalopathy is particularly important because of the brief therapeutic window and possible side effects of neuroprotective intervention [1].
HIE is clinically defined as a syndrome of disturbed neurological function in neonate after birth, manifested by difficulty with initiating and maintaining respiration, depression of tone and reflexes, subnormal level of consciousness and seizures. Neonatal encephalopathy that results from systemic hypoxemia and decreased cerebral perfusion leading to ischemia is termed HIE [2].
The fate of birth asphyxia may include death or other complication like seizures, epilepsy, cerebral palsy and developmental delay. The incidence is higher in infants of diabetic or toxemic mother, intrauterine growth retardation (IUGR) and breech presentation as the previous events are associated with increased incidence of asphyxia [3].
Based on the previous studies and clinical experience therapeutic window in human neonatal HIE seems to be within 6 hours after birth. Thus, it is important to look for useful predictors early in the course of the disease [4].
Nucleated red blood cells count per 100 white blood cells in umbilical venous blood of newborn has been reported as a simple, quick and cheap marker of prenatal asphyxia, based on the fact that hypoxic events induce fetal compensatory response in the form of exaggerated erythropoiesis and influx of immature red blood cells into fetal circulation [5].
Lactate is produced in the event of hypoxia and poor tissue perfusion. Any reduction of oxygen and substrate delivery to the fetus, aerobic metabolism through krebs cycle cannot be sustained and tissue need anaerobic metabolism to meet energy requirement, this in turn leads to increase in the production and accumulation of blood lactate [6].
Blood lactate concentration in critically ill and injured patients can be used to detect tissue hypoxia at an early stage which is simple, cheap and quick marker to predict and asses illness severity and outcome [7].
This is a prospective case control study conducted on 60 HIE neonates delivered either vaginally or by cesarean section in Tanta University Hospital during the period of twelve months from January 2017 to December 2017. These were compared to 60 neonates matched in age, sex and body weight apparently healthy term neonates as a control group
Both groups of patients and control were subjected to Full maternal history taking, full detailed history of resuscitation, Apgar score at 1 and 5 minutes. System examination with special emphasis on neurological examination with assessment of severity of hypoxic ischemic encephalopathy using Sarnat and Sarnat staging [9].
All case were subjected to umbilical cord sampling at time of birth, 5 cc of umbilical cord blood were taken and divided as follow: 2 cc for CBC, and Nucleated RBCs, 2 cc for Lactate and electrolyte and 1 cc for arterial blood gas
The data were coded, entered and processed on computer using SPSS (latest version). The level P < 0.05 was considered the cut-off value for significance.
This study was conducted on neonates delivered at Tanta University Hospital during the period of 12 months from January 2017 to December 2017. This study was conducted on 2 groups:
Table 1 showed that there was no statistical significant difference between both groups as regard the sex (P value > 0.05). There was no statistically significant difference as regarding weight and gestational age (p value > 0.05). There was significant low Apgar score in HIE neonates in relation to the controls (P value < 0.0001).
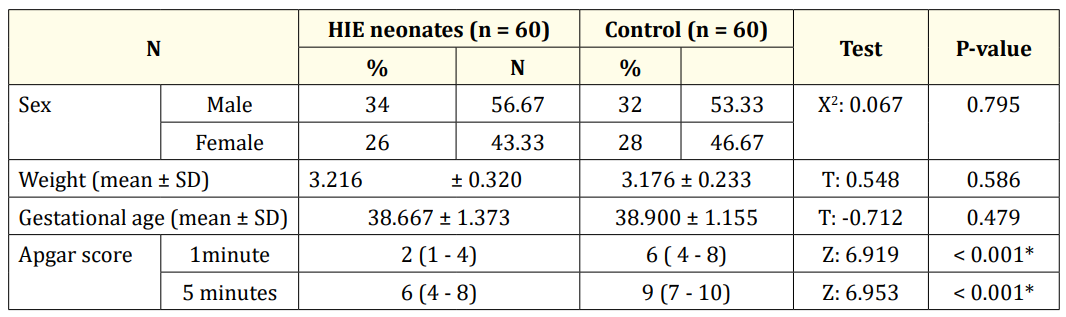
Table 1: Demographic data and Apgar score of HIE and control newborn infants.
Table 2 showed that patients were divided according to Sarnat and Sarnat scoring system into three stages, 30% were classified as grade Ι HIE, 30% as grade Π HIE and 40% as grade Ш HIE.
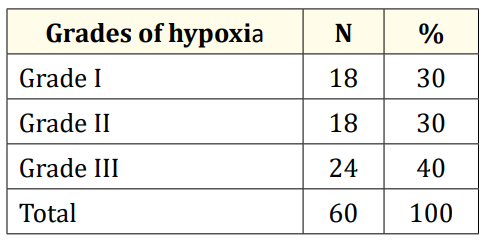
Table 2: Classification of HIE neonates (group 1) according to grades of hypoxia.
Table 3 showed that there was a highly significant difference between both groups in relation to nucleated red blood cells and serum lactate level(higher in HIE than in control) (P value < 0.001).
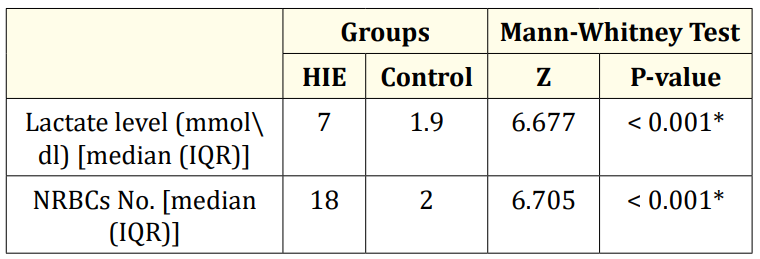
Table 3: Comparison between HIE neonates and control group as regarding nucleated red blood cells and serum lactate level.
Table 4 showed that there was highly significant difference in outcome among different grades of hypoxia (P value < 0.001). It also showed significant difference between levels of serum lactate and nucleated red blood cells (P value < 0.001).
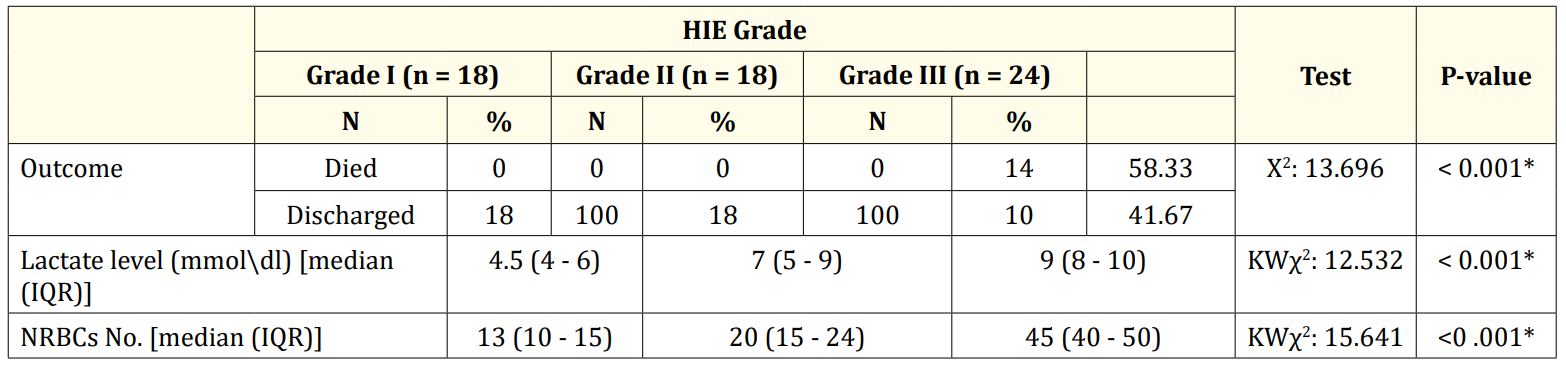
Table 4: Comparison between the three grades of hypoxia as regarding nucleated red blood cells and serum lactate level and outcome.
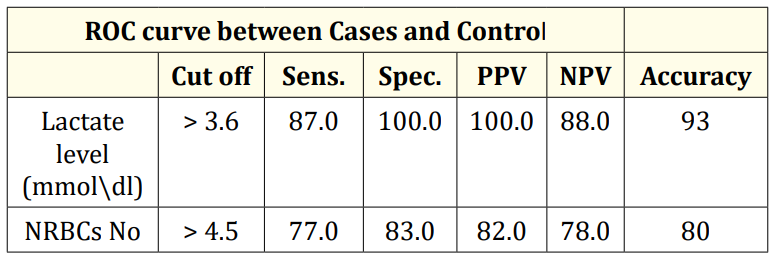
Table 5: ROC curve between Cases and Control.
The sensitivity and specificity were computed by establishing receiver operating characteristic (ROC) curves. The clinical values corresponding to the combination of highest sensitivity and specificity determined at the apex of the (ROC) curves were chosen. An ideal curve of a reasonable test should have the larger area under curve of the ROC graph.
The best cutoff of nucleated red blood cells to detect hypoxia was > 4.5/100 WBCs with a sensitivity of 77%, specificity 83%, PPV 82% and NPV 78% with a diagnostic accuracy 80%.
Serum lactate level were reliable to predict hypoxia as P value < 0.0001 and area under the curve (ROC) was 95% and the best cut off of serum lactate to detect hypoxia > 3.6 mmle/L yielded a sensitivity of 87%, specificity 100%, PPV 100% and NPV 88% with a diagnostic accuracy of 93%.
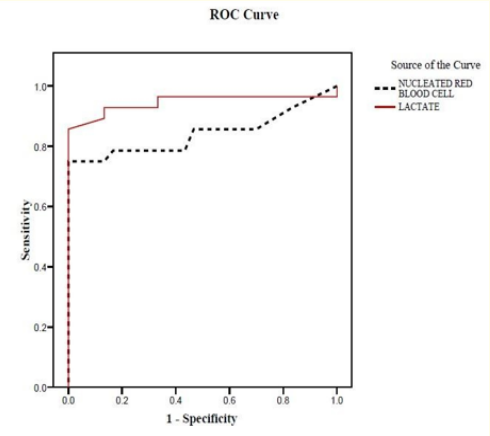
Figure 1: Receiver Operating Characteristic (ROC) curve to define the best cutoff to detect hypoxia.
Hypoxic ischemic encephalopathy after prenatal asphyxia is an important cause of neonatal morbidity, neurological disability and mortality. The early prediction of hypoxic ischemic encephalopathy is particularly important because of the brief therapeutic window and possible side effects of neuro protective interventions [1,10].
In spite of major advances with sophisticated monitoring technology and knowledge of fetal and neonatal pathology, perinatal asphyxia or more appropriately, HIE remains a serious condition, that leaves a significant handicaps in the survivors [11].
In our study the median of Apgar score at 1 and 5 minutes was 2 and 6 respectively and it was significantly lower than the control which had normal Apgar score (7 - 9 at 1 and 5 minutes), in agreement with our study some studied showed that low Apgar score was associated with many problems [12].
In our study the median of nucleated red blood cells in the HIE group was 18⁄100WBCs with a range from 13 - 53 while the median of NRBCs in control group was 2/100WBCs. This in agreement with some studies who found that the median of NRBCs in asphyxiated newborn was higher than the control healthy group [13].
In our study the Levels of nucleated red blood cell per 100 white blood cells were higher in HIE group than in control healthy group this was in agreement with some studies who stated that the Levels of nucleated red blood cell per 100 white blood cells was related to the severity of asphyxia and clinical outcome [14].
In our study the median of serum lactate level in hypoxic group was 7 mmol/L while in the control group the level of lactate was 1.9 mmol⁄dl which was statistically significant as p value < 0.0001. This in agreement with some studies who found that serum lactate was higher in hypoxic group than healthy group [15,16].
In our study the serum lactate level is higher in neonatal hypoxia than the healthy control group and the more grade of HIE the higher serum lactate. This in agreement with some studies who found that serum lactate was higher in neonatal hypoxia with the more sever hypoxia the more serum lactate [17].
In our study nucleated red blood cells could be used with other markers to detect HIE and best cut off of nucleated red blood cells to diagnose hypoxia was > 4.5/100 WBCs with a sensitivity of 77%, specificity 83%, PPV 82% and NPV 78% with a diagnostic accuracy of 80%. This in concordance with some studies who stated that NRBC counts can predict brain injury and neurological outcomes in asphyxiated neonates [18].
In our study serum lactate level to diagnose HIE was > 3.6 mmol/L which yield a sensitivity of 87%, specificity 100%, PPV 100% and NPV 88% with a diagnostic accuracy of 93%.This is in agreement with some studies which concluded that Umbilical lactate can be used in a middle-low resource setting as a measurement of intrapartum hypoxia, with reasonable sensitivity and specificity [19,20].
In conclusion, we found that both nucleated red blood cells and lactate could be used as early predictors in diagnosis of hypoxic ischemic encephalopathy being very easy, cheap and non-invasive measure.
Copyright: © 2018 Mohamed Shawky Elfarargy., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.