Ahmed Anas GUERBOUB1*, Jade ISSOUANI1, Hamza TOUFIK2 and Yassine ERRAHALI1
1Endocrinology and Diabetology Department, Mohammed V Military Academic Hospital, Faculty of Medicine and Pharmacy, Mohammed V- University Souissi, Rabat, Morocco
2Rheumatology department, Mohammed V Military Academic Hospital, Faculty of Medicine and Pharmacy, Mohammed V- University Souissi, Rabat, Morocco
*Corresponding Author: Ahmed Anas GUERBOUB, Endocrinology and Diabetology Department, Mohammed V Military Academic Hospital, Faculty of Medicine and Pharmacy, Mohammed V- University Souissi, Rabat, Morocco.
Received: June 01, 2024; Published: August 26, 2024
Citation: Manoj Kumar Ghosal., et al. “Bone Impact of diabetes: Bone Mineral Density Study”. Acta Scientific Orthopaedics 7.9 (2024): 28-36.
Introduction: Little is known about the impact of diabetes on bone mineral density, and the pathophysiological mechanism differs according to the type of diabetes. With this in mind, we conducted a study to assess this impact in a population of Moroccan diabetics.
Materials and Methods: This is a cross-sectional study conducted in the endocrinology department. The aim was to assess the prevalence of diabetes-related bone damage based on bone mineral density, and to study its relationship with various demographic, clinical and biological parameters in this population.
Results: One hundred twenty-six patients were included in our study. Mean age was 54.19 years, with predominance of type 2 diabetes (71.43%). They were 50.79% male and 49.21% female. The average duration of diabetes was 9 years. Average weight: 76.40kg. Height: 1.66m. FBG: 1.87g/l. HbA1c:8.16%. Body fat percentage: 37.1%. Vertebral fracture was noted in 4.76% of patients, Osteopenia in 35.71% and osteoporosis in 4.76%. Vit D deficiency was noted in 80.15% of patients. A statistically significant correlation was found between BMD T-score and age (p = 0.04), gender (p = 0.05), menopause (p = 0.016), VF (p = 0.47), weight (p = 0.03), height (p = 0.001), % MG (p = 0.034) and BMD at Femur neck (p < 0.001) and lumbar spine (p < 0.001), as well as with vitamin D status (p < 0.001).
Conclusion: It has been established that there is a correlation between diabetes and bone fragility. However, there is currently a lack of knowledge regarding the management of this complication. It is notable that there are currently no recommendations in place for the treatment of osteoporosis in patients with diabetes. Prevention relies on a number of factors, including physical activity, weight loss, fall prevention, particularly in the event of hypoglycaemia, and correction of vitamin D status.
Keywords: Diabetes; Bone; BMD; Osteoporosis; Fracture
Diabetes is a global health problem with far-reaching consequences. The microangiopathic and macroangiopathic complications of diabetes are well studied and detected in diabetic patients, but the consequences for bone are poorly understood. The aim of our work is to assess the prevalence of this bone damage, its associated factors and its relationship with various demographic, clinical and biological parameters in a population of Moroccan diabetic patients.
Our work consists of a cross-sectional study of 126 male and female diabetic patients of all types, aged between 19-81 years.
The main objectives of this study are to assess the bone impact in diabetic patients, and to correlate this with the various demographic, clinical and biological parameters.
Patients were recruited over a 06-month period from January to June 2023 in the endocrinology department of the Mohammed V Military Academic Hospital. Bone densitometry was performed in the rheumatology department, and laboratory tests were carried out in the same hospital.
Diabetic patients followed at the endocrinology department of the Mohammed V Military Academic Hospital were included in the study.
Data were collected using a pre-established data collection form, filled in from the patient’s medical record at the archive or consultation department.
Our data sheet identifies the following elements:
Variables were classified in the Epidemiology and Research Methodology Laboratory at the Faculty of Medicine and Pharmacy, RABAT, using Jamovi (2020) version 1.6 software: qualitative variables were expressed as numbers and percentages. Quantitative variables were expressed as mean standard deviation or median and quartiles, depending on their distribution. Univariate analysis was performed using appropriate statistical tests. The p value was considered significant for a value < 0.05.
One hundred twenty-six patients were included in our study. The mean age was 54.19 years, with a predominance of type II diabetes (71.43%). They were 50.79% male and 49.21% female, of whom 70.97% were menopausal. Average weight = 76.40kg, height = 1.66m. The average duration of their diabetes was 9 years, and FBG = 1.87g/l, HbA1c = 8.16%. Concerning diabetic complications, 50.79% of patients had at least one microangiopathic complication. Of the 64 patients with microangiopathy, 33 had a single microangiopathy, of whom 24 (72.72%) had diabetic retinopathy and 9 (27.27%) diabetic nephropathy. On the other hand, 26 patients had both retinopathy and nephropathy. The presence of all three, diabetic retinopathy, nephropathy and neuropathy, was noted in 5 patients (7.81%). In our series, 61 type 2 diabetic patients were on oral antidiabetic drugs (OADs). Patients on insulin therapy accounted for 33.33%, including 34 T1DM and 8 T2DM. The combination of OADs and insulin was found in 16.67% of patients, including 19 T2DM and 2 T1DM; while only 2 T2DM patients were on dietary hygiene measures.
Bone-wise, the median T-score was 0.05 [(-0.90)-0.60] at the Femur neck (FN) and (-0.30) [(-1.28)-0.60] at the lumbar spine (LS). Mean bone mineral density (BMD) in T1DM and T2DM was (-0.03; -0.07) at the FC and (-0.38; -0.39) at the LS, respectively. In our series, osteodensitometric status was normal in 58.73% of patients (n = 74), osteopenia in 35.71% and osteoporosis in 5.56%, including one patient with severe osteoporosis. BMD at the femoral neck was normal in 78.57% (n = 99) of patients, with a mean T-score = 0.37. Osteopenia was marked in 25 or 19.84% of patients, with a mean of (-1.6), while osteoporosis was found in two patients, including one at the severe osteoporosis stage, with a mean of (-2.7). Among patients with normal BMD at the femoral neck, 69.69% were T2DM with a mean T-score = 0.34 and 30.30% were T1DM with a mean of 0.49; for patients with osteopenia, 76% were T2DM with a mean of (-1.49) and 24% T1DM with a mean of (-1.51). Only two osteoporotic patients were type 2 diabetics, with a mean of (-2.7). BMD at the lumbar spine was normal in 64.29% (n = 81) of patients, with a mean T-score = 0.42. Osteopenia was marked in 38 patients (30.16%) with a mean of -1.58, while osteoporosis was found in seven patients (5.56%), including one at the severe osteoporosis stage, with a mean T-score = -3.34. Among patients with normal BMD at the lumbar spine, 67.90% were T2DM with a mean T-score = 0.45 and 32.09% were T1DM with a mean of 0.37. For patients with osteopenia, 78.94% were T2DM with a mean of (-1.6) and 21.05% were T1DM with a mean of (-1.52). For osteoporotic patients, 71.42% were T2DM with a mean T-score = (-3.4), and 28.57% T1DM with a mean of (-3.2). Vertebral fracture in 4.76% of patients. The demographic, clinical and biological data of our patients are summarized in table 1.
![<p>Table 1: Characteristics of patients studied (n = 126).</p>
<p>* Mean ± standard deviation ** Median [Interquartile] Min: MinimumMax: Maximum</p>
<p>HbA<sub>1</sub> c: Glycated Hemoglobin; FBG: Fasting Blood Glucose; BMI: Body Mass Index; BMD: Bone Mineral Density; FN: Femoral Neck; LS:</p>
<p>Lumbar Spine; HDLc: High-Density Lipoprotein Cholesterol; LDLc: Low-Density Lipoprotein Cholesterol; TG: Triglycerides</p>](https://actascientific.com/ASOR/images/IJMCR/ASOR-07-0983-table1.PNG)
Table 1: Characteristics of patients studied (n = 126).
* Mean ± standard deviation ** Median [Interquartile] Min: MinimumMax: Maximum
HbA1 c: Glycated Hemoglobin; FBG: Fasting Blood Glucose; BMI: Body Mass Index; BMD: Bone Mineral Density; FN: Femoral Neck; LS:
Lumbar Spine; HDLc: High-Density Lipoprotein Cholesterol; LDLc: Low-Density Lipoprotein Cholesterol; TG: Triglycerides
In order to determine the factors affecting BMD in our diabetic patients, we performed a univariate analysis (Tables 2 and 3). Analysis of the densitometric status of our patients showed a positive correlation between BMD T-score and weight (p = 0.03), height (p = 0.001), BMD at FN and LS (p < 0.001). Their levels decreased with the onset of osteoporosis.
![<p>Table 2: Comparison of quantitative patient characteristics according to BMD T-Score.</p>
<p>Mean ± standard deviation** Median [Interquartile range] * Mean ± standard deviation** Median [Interquartile range] * Standard deviation BMD: Bone Mineral Density; FN: Femoral Neck; LS: Lumbar Spine; FBG: Fasting Blood Glucose HbA1c: Glycated Hemoglobin; BMI: Body Mass Index; HDLc: High Density Lipoprotein Cholesterol; LDLc: Low-Density Lipoprotein Cholesterol; TG: Triglycerides</p>](https://actascientific.com/ASOR/images/IJMCR/ASOR-07-0983-table2.PNG)
Table 2: Comparison of quantitative patient characteristics according to BMD T-Score.
Mean ± standard deviation** Median [Interquartile range] * Mean ± standard deviation** Median [Interquartile range] * Standard deviation BMD: Bone Mineral Density; FN: Femoral Neck; LS: Lumbar Spine; FBG: Fasting Blood Glucose HbA1c: Glycated Hemoglobin; BMI: Body Mass Index; HDLc: High Density Lipoprotein Cholesterol; LDLc: Low-Density Lipoprotein Cholesterol; TG: Triglycerides
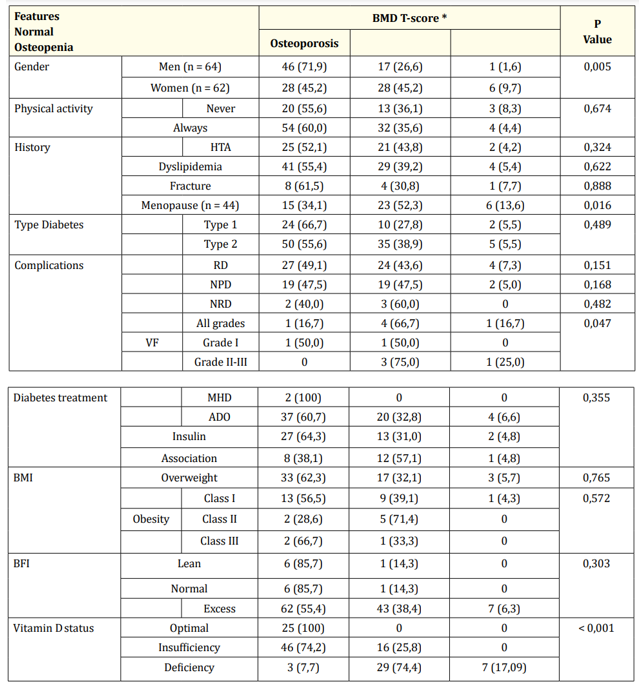
Table 3: Comparison of qualitative patient characteristics by densitometric status.
*Number (Percentage%)
BMI: Body Mass Index;BFI: Body Fat Index DR: Diabetic Retinopathy; DNR: Diabetic Neuropathy; DN: Diabetic Nephropathy; VF: Vertebral Fractures
There was a statistically significant relationship between densitometric status and gender (p = 0.005) and menopause (p = 0.016). Men were less affected by osteopenia and osteoporosis (26.6%; 1.6%) than women (45.2%; 9.7%); and menopause was noted in all osteoporotic women.
There was also a negative correlation between BMD T-score and age (p = 0.04), and % BFI: Body Fat Index (p = 0.034). Patients with osteoporosis are older and have higher body fat.
Densitometric status is linked to the prevalence of VF (p = 0.47), predominantly grade II-III, (75%) for osteopenia and (100%) for osteoporosis. It is also related to vitamin D status (p < 0.001): the more patients suffer from osteopenia and osteoporosis, the more they suffer from insufficiency and deficiency.
There was no statistically significant relationship between BMD T-score and other demographic, clinical and biological characteristics of patients.
Diabetes has an impact on bone, with an increased risk of fracture. This has been demonstrated in several studies. But the extent of this damage and its associated factors vary from study to study. We therefore conducted a study on a case series of Moroccan diabetic patients. The aim was to assess the prevalence of bone damage, its associated factors and its relationship with various demographic, clinical and biological parameters in this population.
Diabetic patients in our series had a mean BMD of (-0.30) at the lumbar spine, and 0.05 at the femoral neck. Bone densitometry revealed osteopenia in 35.71% of patients, and osteoporosis in 5.55%, all sites combined. BMD levels at LR and CF were significantly associated with the prevalence of osteopenia and osteoporosis (p < 0.001).
In the literature, osteoporosis is the most important metabolic bone disease in patients with diabetes mellitus [1]. A study of a prospective cohort of 32,089 postmenopausal women in the Iowa Women’s Health Study found that women with type 1 diabetes mellitus (T1DM) were 12 times more likely to report hip fractures than women without T1DM. However, women with type 2 diabetes mellitus (T2DM) were also 1.7 times more likely to report hip fractures than women without T2DM [2].
The prevalence of osteopenia and osteoporosis varies considerably in patients with T1DM. Studies on the prevalence of osteoporosis in patients with T1DM can be classified as follows
Studies of children or adolescents assessing BMD in a growing skeleton with recent onset of T1DM. Such as the study by Valerio (2002) [3] where BMD LR was lower (mean Z-score, -0.44 ± 1.02) in Italian diabetic adolescents compared with 43 non-diabetic controls, with a negative correlation between BMD LR Z-score and age, disease duration and glycosylated hemoglobin.
These were middle-aged patients with long-standing diabetes and, more often than not, associated diabetic complications. The results were controversial. For example, Rozadilla’s (2000) study [4] found a decrease in LR BMD (Z score -0.32), non-significant decrease in CF BMD in T1DM, osteoporosis present in 3%. Retinopathy associated with low BMD. While Bridges (2005) [5] found no differences in distal Radius BMD between T1DM, T2DM patients and normal controls.
With regard to T2DM, the Rotterdam study [6], the largest study of BMD in T2DM, included BMD and fracture data from 792 elderly T2DM patients (483 women and 309 men; mean age: 74 years) and 5863 non-diabetic controls, and confirmed that the presence of treated T2DM carries an increased risk of fracture hazard ratio: 1.33; 95% CI. A subset analysis revealed an increased fracture risk only in treated T2DM patients (hazard ratio: 1.69; 95% CI, 1.162.46), but a lower fracture risk in patients with glucose intolerance (hazard ratio: 0.80; 95% CI, 0.63-1.00).
Similarly, Strotmeyer., et al. [7]. evaluated 566 patients (243 women and 323 men) with T2DM in their mid-sixties and reported a 4-5% higher BMD at the hip, independent of sex and race. A smaller, all-female population with a mean age of 75 showed BMD values 11% higher at the femoral neck and 8% higher at the lumbar spine in women with T2DM compared with healthy controls [8]. Other studies involving younger patients have confirmed these results.
In a Korean study evaluating 185 women with T2DM, lumbar spine BMD was slightly higher than that of a healthy, age-matched control group, and BMD values were negatively correlated with age (r -0.58), years since menopause (r -0.47) and, to a lesser degree, disease duration (r -0.19) [7].
In a study of 65 Italian women with T2DM (mean age: 63 years), the mean T-score for BMD at the femoral neck was -1.62 ± 1.03 compared with -2.24 ± 0.97 in 42 non-diabetic controls, while BMD at the lumbar spine was not significantly different [9]. This study proposed that different skeletal sites may be variably affected by T2DM. Another report suggests that sites predominantly composed of cortical bone, such as the distal radius, may in fact be diminished, in Japanese patients with T2DM, mean T-scores were 0.8 lower in 64 men and 1.1 lower in 81 women compared with 95 non-diabetic controls [10].
In our study, the association between VF and BMD (T-score) (all sites combined) was borderline significant (p = 0.047). We thus note an increase in the prevalence of these fractures with the onset of osteopenia. Separately, a higher prevalence of VF (p = 0.32) was associated with a higher BMD (T-score) at LS (50% vs. 16.7%), in contrast to the study by Napoli N et al. where a higher spine BMD was associated with a lower prevalence of VF in diabetic men (p = 0.24) [11].
On the other hand, a high prevalence of VF was associated with a decreased BMD (T-score) at FN (p < 0.001) similar to a Brazilian study by Lopes et al. showing that BMD at FN correlated with VF [12], and El Maghraoui et al. determined BMD at FN as a risk factor for VF and not BMD at LS suggesting established effects of aging on the spine that may falsely increase BMD measurement at this site [13].
In our series, age correlated with BMD (T-score) (p = 0.04), and our patients suffering from osteopenia and osteoporosis were older but with a lower prevalence of the latter, thus corresponding to the study by El Maghraoui a. and colleagues on post-menopausal Moroccan women, which demonstrated that osteoporotic patients were older than non-osteoporotic ones (p < 0.0001) [14].
In this sense, the study by Alexopoulou O et al. reported that age was positively correlated with densitometric status (p = 0.018) and that osteoporosis was present in only a minority of subjects, suggesting that osteopenia in diabetic individuals may be attenuated with increasing age [15].
More men have a normal densitometric status, and are less affected by osteopenia and osteoporosis (71.9%; 26.6%; 1.6%) than women (45.2%; 45.2%; 9.7%). Alexopoulou O et al. also report this discrepancy in their study concluding that osteopenia remains a more frequent complication of diabetes than osteoporosis in male subjects [15].
The study by Nicodemus KK, Folsom AR reports that postmenopausal women with type 1 diabetes (n = 47) were 12.25 times more likely to report a hip fracture than women without diabetes; and women with type 2 diabetes had a 1.70 times greater risk of hip fracture than women without diabetes. Postmenopausal women with diabetes, or in whom diabetes develops, have a higher risk of hip fracture than postmenopausal women without diabetes [2].
In our study, menopause was statistically associated with BMD (T-score) (p = 0.016), and the duration of menopause in women with osteoporosis was longer. This was consistent with the study by El Maghraoui A., et al., which demonstrated that patients with osteoporosis have a longer duration since menopause (p < 0.0001) [14].
In our study, T2DM (3.96%) predominated among osteoporotic patients, compared with T1DM (1.59%). The mean BMD (T-score) in T1DM and T2DM were at LR (-0.38; -0.39) and FC (-0.03; -0.07) respectively.
Except that studies report decreased BMD levels during T1DM, for high or even normal levels during T2DM, such as P Vestergaard’s study, which demonstrated that the BMD Z-score was decreased in the spine (-0.22 ± 0.01) and hip (-0.37 ± 0.16) in T1DM, and increased in the spine (0.41 ± 0.01) and hip (0.27 ± 0.01) in T2DM [16]. Our results do not agree with this study in the sense that FN has higher BMD rates than LS irrespective of diabetes type. The discrepancy between the reports and our study regarding the decrease in BMD during T2DM may be due to several factors, including differences in BMD measurement methods, study design and patient selection.
It has been suggested that long-standing T2DM may predispose to a higher incidence of falls, thus increasing the likelihood of suffering fractures, despite a higher mean BMD in these patients [1].
Observations by Mc Nair et al. showed that the rate of bone loss was significantly higher in insulin-treated patients with diabetes for 1 to 6 years (n = 29, bone loss: 1.96 ± 0.32%) than in patients with a longer duration of the disease (n = 31, bone loss: 0.61 ± 0.44%), and thus bone loss develops mainly during the first years of diabetes (P < 0.05) suggesting a protective effect of insulin therapy [17].
In the study by Campos et al. in T1DM, the prevalence of osteopenia and osteoporosis was at baseline (n = 62; 44%; 26%) in at least one site, and after 7 years on treatment was (n = 57; 32%; 34%) (P < = 0.001). This confirms the negative effect of disease duration on BMD [18].
The results of our study concur with those of Campos et al. given that the average age of diabetes in our osteoporotic (16 years) and osteopenic (10 years) patients compared with those of normal densitometric status (8 years), and thus the progression to osteoporosis with the evolution of diabetes over time.
Several studies suggest that overweight protects against bone loss. The study by Alexopoulou O. et al. demonstrated a positive correlation between BMD Z-score at the lumbar spine and left hip, and weight (p < 0.05) [15].
El Maghraoui a. et al. in their study showed that patients with osteoporosis had less weight and height (p < 0.0001), BMI (p = 0.017) and total body fat percentage [14] suggesting a protective effect of adiposity. Our results are almost in line with this, where BMD (T-score) was significantly related to weight (p = 0.003), height (p = 0.001) and % body fat (p = 0.034). Our patients with osteoporosis had less weight and height and a low BMI, but more body fat.
The study by Lili et al. showed that higher BMI was positively associated with higher BMD levels (p < 0.05) in diabetics. Thus, body fat may have an impact on BMD levels. Indeed, adipose tissue releases a wide variety of adipokines directly or indirectly involved in regulating bone remodeling. Plasma leptin concentrations were found to be higher in diabetic men than in healthy controls, while circulating adiponectin levels were reduced [19]. Similarly, Alexopoulou [15] in his study found that leptin values were related to BMD Z-score at the lumbar spine and left hip (r = 0.343; P = 0.03 vs r = 0.395; P = 0.012); and the study by Lenchik L. et al, demonstrated that BMD was inversely associated with serum adiponectin (r 0.20 to 0.3; P < 0.01) and % body fat (p < 0.0001) in patients with T2DM, but not related to BMI [20].
In our analysis, densitometric and vitamin D status were positively correlated (p < 0.001). Patients with osteopenia or osteoporosis were respectively deficient (35.5%) or deficient (64.4%) in vitamin D. Patients with VF were also found to be deficient and deficient in vitamin D (66.7%; 33.3%), and none had normal status.
Similarly, Lopes et al. reported in their study higher serum 25OHD levels in the non-fracture group than those observed in the fracture group, and vitamin D insufficiency was more observed in this group (93.65% vs. 82.3%, p = 0.001), highlighting vitamin D insufficiency as a contributing factor in moderate/severe vertebral fractures [12].
El Maghraoui., et al. compared post-menopausal patients according to vitamin D status and found that osteoporosis was not associated with hypovitaminosis D, although patients with vitamin D deficiency or insufficiency compared to those with normal status had higher prevalences of osteoporosis (28.3%; 25%; 16%). A significant association between Vit D status and T-scores at the lumbar spine and total hip (p = 0.001) was marked. In the same study, vitamin D status was correlated with the prevalence of vertebral fractures (p = 0.002). Thus, they conclude that vitamin D status is an important factor in the presence of VF, and patients in deficiency had twice the risk of osteoporotic fractures, and suggest that serum vitamin D concentrations could be a useful predictor of hip fracture risk in the elderly given its association with BMD at FN [13].
Diabetes mellitus and osteoporosis are two common conditions, with increasing prevalence in the aging population. Patients with T1DM are at high risk of osteoporotic fractures. The initial period of insulin deficiency leads to impaired bone formation. Poor glycemic control in long-term disease is associated with retinopathy, peripheral neuropathy, nephropathy and peripheral vascular disease, which are predictive of low bone mass and increased fracture risk. Prevention and appropriate therapeutic management of these complications, screening for low BMD and awareness of other associated diseases (e.g. celiac disease) are recommended in patients with T1DM. In contrast, patients with T2DM have an increased risk of fracture despite a higher BMD, which is mainly due to the increased risk of falls. Thus, normal BMD values can be misleading. Adequate glycemic control and prevention of diabetic complications are also the mainstay of treatment for T2DM. In addition, risk factors for falls (advanced age, balance disorders, cardiovascular disease, neuropathy) must be identified and minimized by implementing a program combining regular exercise, vitamin D supplementation, withdrawal of psychotropic medication, visual assessment, and evaluation and modification of environmental risks.
All authors declare that they have no conflicts of interest.
Copyright: © 2024 Ahmed Anas GUERBOUB., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.