Jacob Birdsall1*, Steven Siarakas1,2 and John Merlino1,2
*Corresponding Author: Jacob Birdsall, Department of Microbiology and Infectious Diseases, Concord Repatriation General Hospital, NSW Health Pathology, Australia.
Received: March 03, 2021; Published: April 07, 2021
Objectives: To compare the fosfomycin minimum inhibitory concentrations of Enterobacterales and non-Enterobacterales, as determined by the BD Phoenix M50, to EUCAST and CLSI clinical breakpoints.
Methods: 158 Gram-negative organisms isolated from clinical samples underwent susceptibility testing using the BD Phoenix M50, NMIC-404 panel. The fosfomycin MICs of both Enterobacterales and non-Enterobacterales were compared to EUCAST and CLSI clinical breakpoints.
Results: A total of 138 Enterobacterales were tested, including 81 E. coli isolates. 131 (95.0%) were considered susceptible using EUCAST breakpoints and 134 (97.1%) were considered susceptible using CLSI breakpoints. Of the 20 non-Enterobacterales tested, 5 isolates (25.0%) showed an MIC below ≤32 mg/L (EUCAST breakpoint for Enterobacterales), and 16 isolates (80.0%) had an MIC of ≤64 mg/L (CLSI breakpoint for E. coli isolates).
Conclusions: This study showed high susceptibility rates of most Enterobacterales to fosfomycin, even in the presence of resistance mechanisms (ESBL and CPO), while most of the non-Enterobacterales tested showed elevated MICs to fosfomycin.
Keywords: coli; Enterobacterales; EUCAST; Fosfomycin
Fosfomycin is a broad-spectrum antibiotic commonly used in UTIs. It has activity against most Enterobacterales, notably Escherichia coli, Proteus mirabilis and Citrobacter spp., while more varied activity has been described against non-Enterobacterales [1]. Mowlaboccus., et al. performed a study which showed low incidence of fosfomycin resistance in Escherichia coli urinary tract infection isolates in Australia (2 of 1033 isolates resistant) [2].
Fosfomycin acts by inhibiting the MurA enzyme, which catalyses the first stage in peptidoglycan synthesis [3]. EUCAST clinical breakpoints for Enterobacterales in uncomplicated urinary tract infection (UTI), are MIC of ≤32 mg/L (susceptible) and MIC of >32 mg/L (resistant), with no other MIC breakpoints or zone diameters set for non-Enterobacterales [4,5]. CLSI clinical breakpoints, which apply only to E. coli urinary tract isolates, are MIC of ≤64 mg/L (susceptible); MIC of 128 mg/L (intermediate); MIC of ≥256 mg/L (resistant) [8].
A previous study examined the accuracy of the Phoenix in detecting fosfomycin resistance compared to other methods (i.e. disc diffusion, Etest, Vitek). Despite the current gold standard for susceptibility testing being agar dilution and disc diffusion, the Phoenix showed 99.5% categorical agreement for E. coli, and 93% for K. pneumoniae, with significantly lower major/very major errors compared to the other methods [7].
Over a three-month period, a total of 158 Gram-negative organisms isolated from clinical samples, were collected. This included 23 extended-spectrum β-lactamase (ESBL) producing organisms and 5 carbapenemase-producing organisms (CPO) (IMP4: n = 2, KPC: n = 1, oxa48: n = 1, AIM: n = 1). Each isolate had susceptibility testing performed on a pure culture using the BD Phoenix M50, NMIC-404 panel. This data was correlated and fosfomycin MICs of both Enterobacterales and non-Enterobacterales were compared to EUCAST and CLSI clinical breakpoints.
A total of 138 Enterobacterales were tested, 81 of which were E. coli. Of the E. coli isolates, 81 (100.0%) were classified as susceptible, including 18 ESBL positive isolates, when EUCAST and CLSI breakpoints were used.
Of the other Enterobacterales (n = 57), 50 (87.7%) were classified as susceptible (MIC of ≤ 32 mg/L) including 5 ESBL producing organisms and 4 CPO, while 7 (12.3%) were classified as resistant using EUCAST breakpoints (MIC of > 32 mg/L); the majority of these were Morganella morganii. Using CLSI breakpoints, 53 isolates (93.0%) were classified as susceptible (MIC of ≤64 mg/L), while 4 isolates (7.0%) showed an MIC of >64 mg/L, which were classified as either intermediate (MIC of 128 mg/L) or resistant (MIC of ≥256 μg/mL); all of which were M. morganii (Figure 1).
Figure 1: Fosfomycin MIC distribution of tested isolates.
A total of 20 non-Enterobacterales were tested, comprised of Pseudomonas aeruginosa (n = 19) and Pseudomonas putida (n = 1). Using EUCAST breakpoints set for Enterobacterales, only 5 isolates (25.0%) showed an MIC below the breakpoint of ≤ 32 mg/L, while 15 isolates (75.0%) had an MIC > 32 mg/L. Using CLSI breakpoints, 16 isolates (80.0%) had an MIC of ≤ 64 mg/L, including 1 CPO, while 4 isolates (20.0%) had an MIC > 64 mg/L (Figure 1).
Of the 23 ESBL producing organisms tested, 23 (100.0%) had an MIC of ≤ 32 mg/L, classifying them as sensitive using both EUCAST and CLSI, with 22 (95.6%) having an MIC of ≤ 16 mg/L. Of the 4 CPO positive Enterobacterales, 4 (100.0%) had an MIC ≤ 32 mg/L classifying them as susceptible according to EUCAST and CLSI (Table 1).
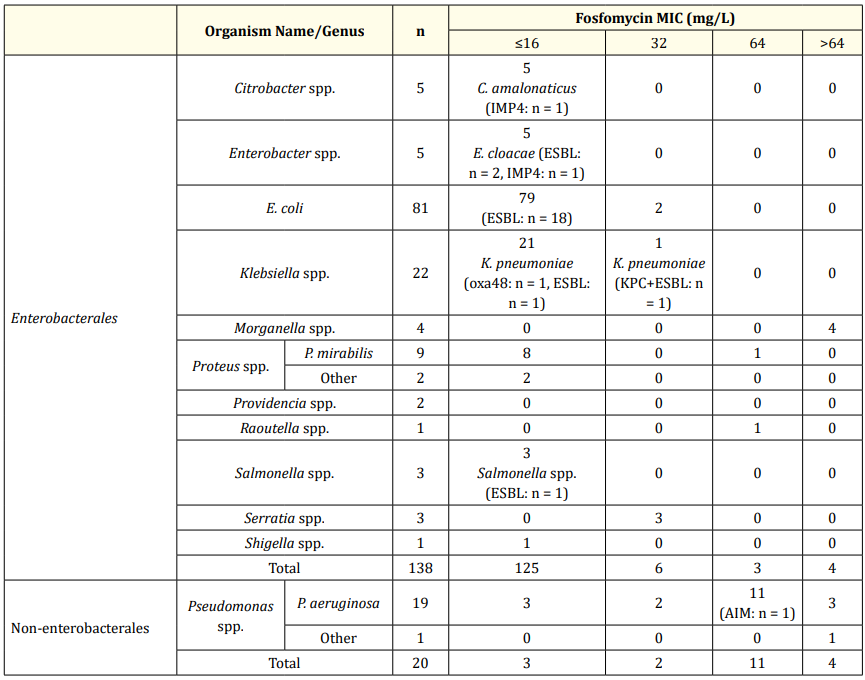
Table 1: Breakdown of tested isolates and respective fosfomycin MIC distribution
Although 100% of the Klebsiella isolates which were tested had an MIC of ≤ 32 mg/L, indicating in vitro susceptibility, reduced clinical activity, emergence of resistance and failure in treatment has been shown to be significant among Klebsiella spp [6,7].
The results of this study showed high susceptibility rates of most Enterobacterales to fosfomycin (excluding Morganella spp.), even in the presence of presumed resistance mechanisms, while most of the Pseudomonas spp. tested showed elevated MICs to fosfomycin. EUCAST and CLSI have not set clinical breakpoints for Pseudomonas spp. Fosfomycin may be a useful therapeutic option for most Enterobacterales, including ESBL and CPO positive isolates, however further study is required to ensure adequate clinical activity. Fosfomycin is unlikely to be beneficial in the treatment of non-Enterobacterales.
None to declare.
The authors state that there are no conflicts of interest to disclose. No funding was received for this study.
Citation: Jacob Birdsall., et al. “Minimum Inhibitory Concentration and Susceptibility Patterns of Organisms to Fosfomycin as Determined by BD Phoenix M50”. Acta Scientific Microbiology 4.5 (2021): 19-22.
Copyright: © 2021 Jacob Birdsall., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.