Nisha Raj1, Ashok Kumar2, Praveer Rai3 and Ram Nawal Rao4*
1Department of Pathology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India
2Department of Surgical Gastroenterology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India
3Department of Gastroenterology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India
4Professor, Department of Pathology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India
*Corresponding Author: Ram Nawal Rao, Professor, Department of Pathology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India.
Received: February 11, 2021; Published: March 16, 2021
Keywords: Gastric Adenocarcinomas (GAC); Mucin-1(MUC1); Immunohistochemistry (IHC); Haematoxylin and Eosin (H&E)
According to Globocan [1], over one million new cases of gastric cancer were estimated to have occurred in 2018, making it the fifth most common malignancy and third leading cause of cancer mortality (8% of all cancer deaths), mainly because of advanced stage at diagnosis. The most common histological subtype is Gastric adenocarcinoma (GAC). Adenocarcinoma comprises 95% of the total number of gastric malignancies. Despite the decline in the overall incidence of gastric cancer (GC) in recent decades, it still represents the second leading cause of cancer related death [2].There is a wide geographical variation in both patterns of incidence and survival [3]. Highest mortality rates have been reported in East Asia, including Japan, Korea and China (28.1 per 1000,000 males, 13.0 per 100,000 females) [4]. More than 21,000 new cases of gastric cancer are reported with more than 10,000 deaths annually in United States [5]. In India, the number of new gastric cancer patients is approximately 34000 each year with male to female ratio of 2:1 [6]. In recent years, efforts have been focused on identifying better molecular targets for treatments that interfere with the signaling cascades involved in cell differentiation, proliferation and survival.
Mucins are high molecular weight glycoproteins expressed by specialized epithelial cells lining the luminal surface of different organs like breast, pancreas, respiratory, gastrointestinal and reproductive tracts. Mucins proteins are well known for providing protection and lubrication to epithelial surfaces. In addition, their roles in cell signaling are under intense study [7,8]. Variations in mucin expression and glycosylation are associated with cancer development [9]. Atypical expression of mucins is likely associated with cancer biology as alterations in the glycosylation patterns influence cellular growth, differentiation, transformation, adhesion, invasion and immune surveillance [10].
It has been reported that MUC1 increases invasion and metastasis in various cancers. The role of MUC1 in both transformation and metastatic progression has led to extensive focus on this protein for the development of targeted therapies to treat metastatic diseases. Several study reported that MUC1 is a poor prognostic indicator, whereas other reports failed to find its association with different clinicopathological parameters [11-15].
There are still controversies between results of MUC1 expression and their association with different prognostic factors [16-20] and limited number of studies are available worldwide on MUC1 status in gastric cancer patients [21-24]. The objective of the present study was to identify and evaluate the frequency of immunohistochemical expression of MUC1 in gastric adenocarcinoma and to evaluate their correlation with the various clinical and histopathological factors.
This was a prospective study of 70 histologically proven patients of gastric or gastro esophageal junction adenocarcinomas who underwent surgery between 2015 to 2018 at Sanjay Gandhi postgraduate institute of medical sciences, Lucknow, India, a tertiary care referral hospital. None of the patients had undergone prior preoperative radiation, chemotherapy or targeted therapy.
Histopathological studyAll specimens were fixed in 10% buffered formalin, within 30 minutes after resection, and for a fixing time of 8-48 hours. Tissues were processed and embedded in paraffin. Tissue blocks were cut in 3-5µ thick sections, fixed for 2hrs at 60˚C. Deparaffinised sections were stained with haematoxylin and eosin. Histopathological diagnosis were made and adenocarcinomas were classified according to Lauren classification into intestinal, diffuse and mixed type. All the clinical data including demographic information (age, gender, symptoms and sign) were retrieved from patients file and from the hospital informatics system prospectively. In addition, histologic type, degree of differentiation, depth of tumor invasion, presence of lymphovascular, perineural invasion and lymph node metastasis were recorded on a predesigned proforma.
The TNM stage of all studied patients was done according to 8th edition of AJCC. Tumors were graded into well differentiated, moderately differentiated and poorly differentiated as per WHO grading system.
ImmunohistochemistryRespective paraffin blocks containing representative samples of the tumors were selected by reviewing H&E stained slides. Paraffin-embedded samples were cut in 3-5μ thick sections, taken on poly L lysine coated slides, fixed for overnight at 60ºC, and then deparaffinized in xylene and hydrat¬ed in a decreasing series of alcohol. After those steps, the endogenous peroxidase was blocked with 3% hydrogen peroxidase. Antigen retrieval was performed in a microwave oven for 30 minutes at 98˚C with the slides immersed in TRIS-EDTA (pH-9). Immunohistochemical staining was performed using Rabbit polyclonal antibody for MUC1 (dilution, 1:100; catalog no., RB9222-P, Thermofisher Scientific, Fremont, CA, USA) followed by addition of diaminobenzidine (DAB) as a chromogen. The slides were stained with hematoxylin for counterstaining and then mounted using DPx. Negative controls (omission of primary antibody) i.e. instead of primary antibody 3% skimmed milk were used. Positive controls were breast carcinoma tissue and gastric cancer tissue (known IHC MUC1, 3+ staining intensity) were used.
Evaluation of IHC resultsImmunohistochemical evaluation was performed according to testing protocol, taking incomplete or complete cytoplasmic and luminal staining. Slides were scored by a two pathologist using the standard scoring system defined earlier (Hwang I., et al. 2012) table 1. Immunostaining scores were independently evaluated in each tumor core.
| Score | Staining Pattern | MUC1protein expression |
|---|---|---|
0 |
0% of tumor cells |
Negative |
1+ |
<10% of weak Positive tumor cells |
Negative |
2+ |
10-50% of mild to moderate Positive tumor cells |
Equivocal |
3+ |
≥50% of strong Positive tumor cells |
Positive |
Table 1: Scoring method for MUC1 immunochemistry in gastric cancers.
Statistical analysis was performed by using Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) 20 package program and results were expressed as mean ± SD, median (IQR) and in percentage. The MUC1 immunohistochemical expression and its correlation with different parameters was evaluated by using chi-square test and Fischer exact test. P value less than 0.05 were considered statistically significant.
Out of seventy patients enrolled in the study there were 43(61.4%) female and 27(38.6%) females, with a mean age of 54.71 ± 1.78 years. As per the site of tumor location is concern maximum number of patients had tumor located in antropyloric lesion 33(63.4%). The second common site of tumor location was at the GE junction 18(25.7%).
The other site of tumor location were body and fundus 8(15.4%), body and antrum 11(21.2%). Patient’s clinicopathological parameters are given in (Table 2).
Tumors were graded into well-differentiated tumors included grade I and moderately differentiated tumors grade II adenocarcinomas; poorly differentiated on the other hand, consisted of grade III adenocarcinomas as per WHO grading system.
According to Lauren’s classification out of 70 cases 40(57.2%) were of intestinal type, 22(31.4%) diffuse type and 8(11.4%) were of mixed type adenocarcinoma. Majority of tumors had moderately differentiated 30(42.8%) followed by poorly differentiated 26(37.2%) and well differentiated tumors 14(20%).Of 70 patients, lymph node metastasis (LNM) was reported in 44(62.9%) patients, while 37(52.8%) had lymphovascular invasion (LVI). Perineural invasion (PNI) was observed in 30(42.8%) patients. The TNM stage of all studied patients was done according to 8th edition of AJCC. Stage III and stage II tumors were observed in 24(34.2%) and 15(21.5%) cases, respectively.
MUC1 IHC scores of patientsMUC1 expression analysis showed that majority of patients 33(47.2%) were of score 3+ suggesting as MUC1 positivity. A score of 2+ was seen in 17 (24.2%) cases i.e. equivocal, while 20(28.6%) cases showed 0/1+ score, which were considered as negative, shown in figure 1.
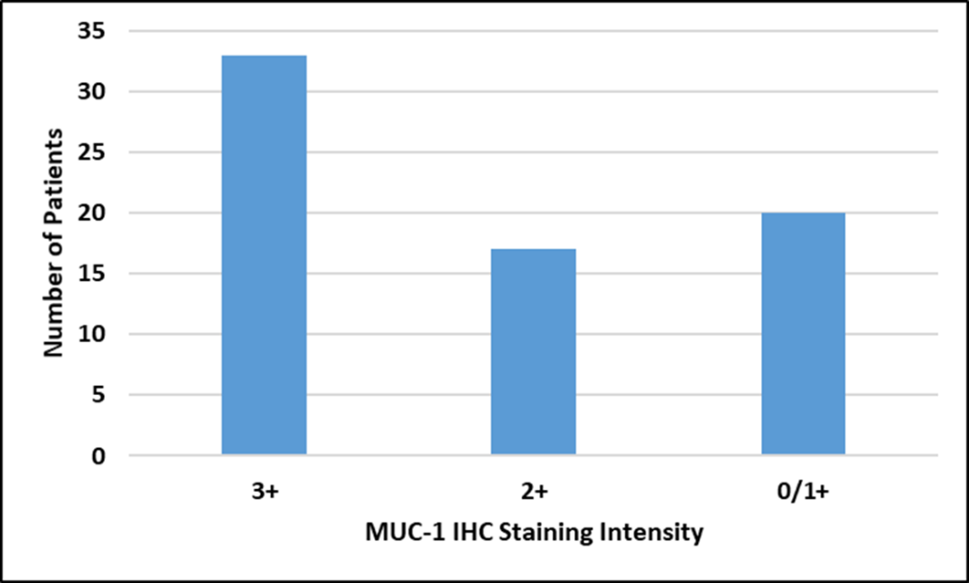
Figure 1: Frequency of MUC1 protein Expression by IHC.
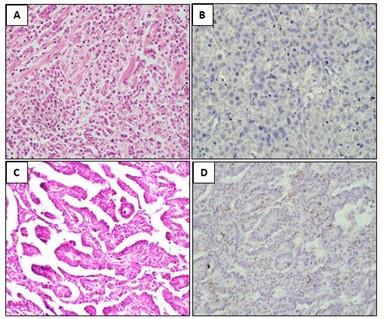
Figure 2: A-B: H&E stain of Signet ring cell adenocarcinoma and corresponding IHC showing no MUC1 staining in tumor cells (score 0, Negative). C-D: H&E Stain of Papillary adenocarcinoma and corresponding MUC1 IHC showing <10% positive staining in tumor cells (score 1+, Negative).
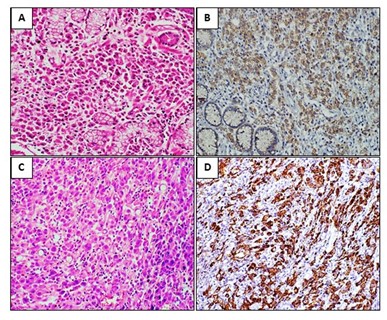
Figure 3: A-B: H&E stain of Moderately differentiated adenocarcinoma and corresponding IHC showing Moderate cytoplasmic MUC1 immunostaining (score 2+, Equivocal) in tumor cells. C-D: H&E stain of Signet ring cell adenocarcinoma and corresponding IHC showing Strong membranous and Cytoplasmic MUC1 immunostaining (score 3+ Strong Positive) in tumor cells.
| S. No. | Clinical Variables | N = 70(%) | MUC-1 IHC Score | X2 | p-Value | ||
|---|---|---|---|---|---|---|---|
| Positive | Equivocal | Negative | |||||
1. |
Gender |
8.83 |
0.012* |
||||
Male |
43(61.4) |
25 |
11 |
7 |
|||
Female |
27(38.6) |
8 |
6 |
13 |
|||
2. |
Age |
7.72 |
0.021* |
||||
>60 |
39(55.7) |
21 |
12 |
6 |
|||
≤60 |
31(44.3) |
12 |
5 |
14 |
|||
3. |
Smoker |
7.26 |
0.026* |
||||
Yes |
45(64.2) |
24 |
13 |
8 |
|||
No |
25(35.8) |
9 |
4 |
12 |
|||
4. |
Tobacco chewer |
1.17 |
0.557 |
||||
Yes |
25(35.7) |
10 |
6 |
9 |
|||
No |
45(64.3) |
23 |
11 |
11 |
|||
5. |
Tumor site |
2 |
0.367 |
||||
Gastric |
52(74.2) |
27 |
12 |
13 |
|||
GE-Junction |
18(25.8) |
6 |
5 |
7 |
|||
6. |
Laurens Classification |
13.1 |
0.010* |
||||
Intestinal |
40(57.2) |
22 |
4 |
14 |
|||
Diffuse |
22(31.4) |
8 |
11 |
3 |
|||
Mixed type |
8(11.4) |
3 |
2 |
3 |
|||
7. |
Tumor Differentiation |
3.43 |
0.486 |
||||
Well |
14(20) |
8 |
4 |
4 |
|||
Moderately |
30(42.8) |
14 |
10 |
7 |
|||
Poorly |
26(37.2) |
11 |
3 |
9 |
|||
8. |
Lymph node metastasis |
6.29 |
0.043* |
||||
Yes |
44(62.9) |
24 |
12 |
8 |
|||
No |
26(37.1) |
9 |
5 |
12 |
|||
9. |
Lymphovascular invasion |
5.31 |
0.070 |
||||
Yes |
37(52.8) |
22 |
8 |
7 |
|||
No |
33(47.2) |
11 |
9 |
13 |
|||
10. |
Perineural invasion |
4.46 |
0.107 |
||||
Yes |
30(42.8) |
18 |
7 |
5 |
|||
No |
40(57.2) |
15 |
10 |
15 |
|||
11. |
Staging |
4.45 |
0.879 |
||||
Stage I |
19(27.2) |
7 |
5 |
9 |
|||
Stage II |
15(21.5) |
6 |
3 |
4 |
|||
Stage III |
24(34.2) |
14 |
4 |
5 |
|||
Stage IV |
12(17.1) |
6 |
3 |
2 |
|||
Table 2: Clinicopathological features and its Correlation with MUC1 Expression.
Values are presented as number (%) and mean ± SD.
MUC1 (Mucin1) oncogene, IHC, Immunohistochemistry.
Correlation analysis- *statistically significant with p-value <0.05.
MUC1 oncoprotein overexpression was seen predominantly in patients over 60 years of age and higher in male than in female population. MUC1 positivity was found more frequently in moderately differentiated tumors 16/30(53.3%) followed by poorly differentiated 11/26(42.3%) and well differentiated tumors 6/14(42.8%). Higher MUC1 positivity 22/40(55%) was reported in the cases with intestinal type as compared to diffuse type 8/22(36.3%) and in mixed type 3/8(37.5%).
Laurens Classification |
No. of Patients |
MUC1 IHC Score |
||
Positive |
Equivocal |
Negative |
||
Intestinal type |
40(57.2) |
22 |
4 |
14 |
Diffuse type |
22(31.4) |
8 |
11 |
3 |
Mixed type |
8(11.4) |
3 |
2 |
3 |
Total |
70 |
33 |
17 |
20 |
Table 3: Correlation of MUC1 Oncoprotein Expression with Histological subtypes.
Cross tabs; Pearson Chi- Square test; p value = 0.010 (Significant).
There was significant correlation between MUC1 positivity and poor prognostic histological subtypes (p = 0.010). In this study no statistically significant correlation of MUC1 expression was observed with tobacco chewer, tumor site, degree of differentiation, lymphovascular invasion (LVI), perineural invasion (PNI) and with TNM staging (p > 0.05).
We found that there was significant difference between MUC1 positive and negative patients in terms of age, gender, smoking, histological subtypes and lymph node metastasis (LNM) (p < 0.05) as listed in table 2.
Gastric adenocarcinoma is a conglomerate of histologically, biologically and genetically diseases, conditioned by the gradual accumulation of various genetic and epigenetic alterations leading to the activation of several molecular pathways. The prognosis of gastric and gastroesophageal junction cancers is still poor despite recent advances in treatment. Although the number of patients diagnosed at early stages is increasing, the majority of patients are still being diagnosed in advanced stages or with metastatic diseases.
The role of new molecular targeted agents is being investigated in an effort to improve the survival rate. The investigation of molecular and genetic changes in gastric cancer has brought new insight into the pathogenesis of disease. In cancer tissues, the expression of MUC1 can be upregulated and expressed on the entire cell surface.
The MUC1 positivity rate in our study was observed in 47.2% of gastric adenocarcinoma cases. However, data reported in literature on MUC1 positivity rates in gastric cancer are variable between 30-60%.Geographical differences, tumor heterogeneity, differences in scoring systems and pathologist expertise may have caused the variations in MUC1 positivity rates between studies. Many studies stated that MUC1 expression was associated with metastatic progression in the gastrointestinal tumors. However, in gastric cancer it was reported that expression of MUC1 was not limited to metastatic disease, but also highly expressed in the majority of isolated cells invading throughout the stroma of primary tumor [19]. Expression pattern of MUC1 in gastric cancer is heterogeneous. This heterogeneity may provide new insights into the differentiation pathways of gastric cancer enabling its use as a clue to bring new insights into biologic behavior of gastric cancer.
Concordance with other reports [25-28], MUC1 showed higher expression rates in intestinal type adenocarcinoma (55%) in which tumor cell interaction is enhanced, than in diffuse type (36.3%) in our study. This finding is supported by the fact that a particular carbohydrate epitope of MUC1 binds to a ligand, cell interactions may be enhanced. Similar to [25] study there was significant difference between MUC1 positive and negative patients in terms of histological subtypes (p = 0.010).
In contrast to previous studies, there was significant difference between MUC1 positivity with age, gender, smoking, histological subtypes and with lymph node metastasis [28,29]. However, there was no significant correlation between MUC1 expression with tobacco chewer, tumor site, degree of differentiation, lymphovascular invasion (LVI), perineural invasion (PNI) and TNM staging. This may be due to the non-binding of ligand with epitope of MUC1. On the other hand, previous studies [10,15,30] stated that there were no significant correlations between MUC1 expression and clinicopathological parameters including Laurens classification and degree of differentiation.
MUC1 staining pattern is different depending on tumor differentiation, stain mainly accumulates at the apex in papillary adenocarcinoma, well differentiated or moderately differentiated adenocarcinoma, while poorly differentiated carcinoma and signet ring cell carcinoma demonstrated primarily cytoplasmic staining [31]. In our study, we could not find significant correlation with MUC1 expression and some prognostic factors such as perineural invasion and TNM staging (p > 0.05).
Our data showed statistically significant correlation between MUC1 positivity and negative patients with age, gender, smoking, histological subtypes and lymph node metastasis (p < 0.05).
MUC1 gene was highly expressed in patients with gastric adenocarcinomas in our study. Significant correlation was found between MUC1 with several clinical and histological factors. Up-regulated MUC1 expression is closely related with progression and it might be considered as a useful prognostic indicator and may give some insight in therapeutic decision-making. However, further more and larger studies are required to validate these findings.
The authors declare that they have no any conflict of interest.
The Institutional Ethical Review Committee (IEC number-2017-21-PhD-95) of Sanjay Gandhi Post Graduate Institute of Medical Sciences in Lucknow, India approved this study.The Institutional Ethical Review Committee (IEC number-2017-21-PhD-95) of Sanjay Gandhi Post Graduate Institute of Medical Sciences in Lucknow, India approved this study.
Informed consent was obtained from all patients included in this study.
The authors thank Indian Council of Medical Research, New Delhi, India for providing fellowship support.
Study concept, design, analysis and interpretation of data: Nisha Raj, Dr. Ram Nawal Rao. The authors would like to express their thanks to Dr. Ashok Kumar for help with advanced research and drafting. Dr. Ram Nawal Rao, Department of Pathology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, India for reviewing the histological and immunohistochemical results. All authors read and approved the final manuscript.
The authors acknowledge the patients for sharing the clinical information and samples.
Citation: Ram Nawal Rao., et al. "MUC1 Expression in Gastric Adenocarcinomas: Its Prognostic Significance and Clinicopathological Correlation". Acta Scientific Gastrointestinal Disorders 4.3 (2021): .
Copyright: © 2021 Ram Nawal Rao., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.