Chibuzor Williams Ifenatuoha*, Ozioma Ebere Chukwudi, Ifeoluwa Victoria Osokomaiya, Precious Ijeoma Chukwudi, Kingsley Obodo and Babatunde Okewale
IVF/Fertility Unit – St. Ives Specialist Hospital, 12 Salvation Rd, Opebi, Ikeja, Lagos, Nigeria
*Corresponding Author: Chibuzor Williams Ifenatuoha, IVF/Fertility Unit – St. Ives Specialist Hospital, 12 Salvation Rd, Opebi, Ikeja, Lagos, Nigeria.
Received: April 03, 2024; Published: September 14, 2024
Citation: Chibuzor Williams Ifenatuoha., et al. “Clinical Pregnancy Outcome in Fresh IVF/ICSI Treatment Between Cleavage-stage (Day 3) and Blastocyst-Stage (Day 5) Embryo Transfer in Post Ovarian Stimulated Patient: A Single-centre Retrospective Analysis". Acta Scientific Women's Health 6.10 (2024):18-23.
Background: There are several reasons behind the choice for the day of embryo transfer. This study addresses the choice of the day of embryo transfer (cleavage-stage or day 3 versus blastocyst-stage or day 5) and the number of embryos transferred in fresh IVF/ICSI treatment, along with their potential effects on clinical pregnancy outcomes in post-ovarian-stimulated patients.
Method: This was a single-centre retrospective study carried out at the IVF/Fertility unit of St. Ives Specialist Hospital, Ikeja-Lagos, Nigeria. The data were retrieved from the electronic medical records of 302 patients who had fresh embryo transfers between 2018 and June 2023. We analyzed the relationship of the day of embryo transfer (D3/D5), the number of embryos transferred and the maternal age to the clinical pregnancy through multiple regression analysis and statistical significance was achieved at p < 0.05.
Result: With respect to the clinical pregnancy, there was a significant difference between day 5 (OR = 4.180, 95% CI: 2.410 to 7.174) and day 3 embryos transfer (OR = 0.2393, 95% CI: 0.1394 to 0.4149) at p <0.0001. However, neither the number of embryos transferred, nor the maternal age significantly influence the clinical pregnancy outcome.
Conclusion: Blastocyst-stage or day 5 embryo transfer has shown a favourable clinical pregnancy outcome than cleavage-stage or day 3 embryo transfer in fresh IVF/ICSI treatment. Also, transferring multiple embryos (blastocyst or cleavage) does not guarantee successful clinical pregnancy outcomes.
Keywords: Fresh Embryo Transfer; Clinical Pregnancy Outcome; Day 3 Embryo Transfer; Day 5 Embryo Transfer
D3: Day 3; D5: Day 5; ET: Embryo Transfer; hCG: Human Chorionic Gonadotropin; hMG: Human Menopausal Gonadotropin; ICSI: Intra Cytoplasmic Sperm Injection; IVF: In-Vitro Fertilization
Infertility is a disease characterized by the inability to conceive or achieve clinical pregnancy after a year of frequent unprotected sexual intercourse [1]. Consequently, this has led to an increasing need for in-vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) treatment. This treatment typically involves the use of hormonal drugs to trigger the ovaries to produce supernumerary oocytes. The oocytes are subsequently collected, fertilized with sperm in the laboratory, cultured in the incubator, and finally the selected embryo(s) will be transferred to the uterus (fresh embryo transfer) or cryopreserved [1,2]. Embryo transfer (ET) can be done at different stages during embryo development after oocyte retrieval. It can be done either on day 2/3 (cleavage stage), on day 4 (morula stage) or 5/6 (blastocyst stage) [3].
Various reasons drive the choice of the day for ET. Historically, as the embryo culture system evolves, there seems to be a simultaneous shift in IVF treatment practice that favours day 5 (blastocyst) ET [2,4]. Even though extending the duration of embryo culture to day- 5/6 may be associated with high cost and safety concerns, blastocyst stage ET closely mimics the fate of an embryo formed during natural conception [3,5]. Similarly, extended embryo culture to day 5 allows the embryos to undergo embryonic genomic activation, which in turn is often related to the selection of good-quality embryos [4,6]. Other additional benefits of blastocyst-stage embryos include a greater implantation chance and a better morphological status that depicts more closely the euploidy state of an embryo [7].
Conversely, extended embryo culture to day 5 is associated with a potential risk, which is the failure of blastocyst formation. Thus, this negatively impacts the blastocyst utilization rate for ET and increases the rate of cycle cancellation [4,7,8]. Moreover, in clinical practices, there is yet to be a consensus on the supremacy of blastocyst over cleavage-stage ET [3].
Several studies do not support fresh embryo transfer due to several reasons. For instance, clinical evidence showed that fresh embryo transfer is not ideal because of its association with ovarian hyperstimulation syndrome (OHSS), adverse perinatal and obstetric outcomes, and low clinical pregnancy rates [6,9]. Nevertheless, some still argue that freezing embryos for frozen embryo transfer (FET) is merely an add-on to the typical IVF treatment, which may not be directly responsible for better maternal and fetal outcomes [9]. However, some clinics that carry out fresh embryo transfer for stimulated patients do so majorly due to patient preference, financial reasons or government funding not covering the freeze-all approach [9,10].
As complete elimination of fresh embryo transfer might not be feasible, our focus lies in comprehending the factors that could potentially influence improve clinical pregnancy in fresh embryo transfers. We aim to investigate whether the day of embryo transfer and the number of embryos transferred has an impact on the clinical pregnancy outcome of fresh embryo transfer among stimulated patients.
This was a single-centre retrospective study. It was conducted at the IVF/Fertility unit of St. Ives Specialist Hospital, Lagos, Nigeria. The study was ethically approved by the local hospital ethical review board. This study was conceptualized based on the hypothesis that blastocyst-stage or D5 ET following oocyte retrieval in stimulated patients will yield better clinical pregnancy outcomes compared to patients who had cleavage-stage or D3 ET. We also hypothesized an increase in pregnancy outcome with multiple embryo transfer on either D3 or D5) compared to single embryo transfer.
This study population included a total of (n = 302) participants who were treated for either primary or secondary infertility. The minimum sample size for the study was determined from Epitools (epitools.ausvet.com.au). it was calculated from the input of the following values: Expected proportion in controls = 0.05, assumed odd ratio = 4, confidence level = 0.95, desired power = 0.8. From the result, a minimum of 98 patients per group will be enough to determine the level of significance between comparable factors in D3 and D5 fresh ET. However, for this study, the data of n = 166 patients who had D3 ET and n = 136 patients who had D5 ET were collected. Patients who had Day 4 (morula stage) ET transfer were excluded.
The data required for this study were retrieved from the IVF/Fertility unit electronic medical records (EMRs). The data of all fresh IVF/ICSI embryo transfers for stimulated patients after oocytes retrievals between February 2018 to June 2023 were collected. For the two groups, D3 ET and D5 ET respectively, patients’ information including the age, number of oocytes retrieved, number of embryos transferred, and clinical pregnancies was collected and documented in a Microsoft Excel spreadsheet.
The data for this study were exclusively gathered from patients who underwent ovarian stimulation and oocyte retrieval procedures. To meet the inclusion criteria, these patients needed to have their embryos transferred either three- or five-days following IVF/ICSI treatment. Conversely, patients who did not undergo ovarian stimulation for oocyte retrieval and instead had fresh embryo transfers with donor oocytes were excluded from the analysis.
The short protocol for ovarian stimulation was used for all patients in this present study. Buserelin (Suprefact) was administered subcutaneously (0.5ml if < 38 years and 0.25ml if > 38 years) daily to patients on the second day of their menstruation for 10 to 13 days (depending on the size of the follicles on ultrasound imaging). Simultaneously, HMG (Menogon – Ferring Global) was administered (3 ampoules daily if between 25 to 38 years and 4 ampoules daily if > 38 years) on the third day of menstruation for 10 days usually. Depending on the sizes of the follicles, hCG is administered on day 12 or 13.
On the scheduled day for oocyte retrieval, 34 to 36 hours after the hCG trigger injection, the cumulus-oocyte complex was retrieved from the ovaries by aspiration under transvaginal ultrasound guidance. After oocyte pick-up in the laboratory, the oocytes were inseminated either by conventional IVF or ICSI. The fertilized oocytes were cultured using SAGE 1-step HSA culture medium overlaid with paraffin oil (Origio) for either up to 3 or 5 days. The cleavage and blastocyst stage embryos were graded based on Gardner’s criteria [11]. Embryo transfer was done either on day 3 or day 5. For day 3 ET, we selected embryos with at least 6 blastomeres and less than 50% fragmentation. Similarly, for day 5 ET, mostly embryos with visible trophectodermal cells and inner cell mass were selected. The ET is generally performed under abdominal ultrasound guidance with the embryos loaded using a Sure-Pro Embryo Transfer Catheter. Clinical pregnancy was confirmed by seeing a gestational sac through abdominal ultrasound.
The data collected were transferred to a Microsoft Excel spreadsheet. The data were exported to GraphPad Prism 9.0.2, where statistical analysis for significance (p <0.05) was conducted.
To identify factors that may be associated with clinical pregnancy, multiple regression analysis was conducted with clinical pregnancy as the independent variable and the maternal age, number of embryos transferred and ET timing (D3/D5) as the dependent variable.
In this study, 302 fresh ET were conducted, of which n = 166 and n = 136 were cleavage stage (D3) ET and blastocyst stage (D5) ET.
The result here shows that the day of ET significantly affects the clinical pregnancy rate (p < 0.0001). Figure 1 shows the comparison of the clinical pregnancy rate between D3 and D5 ET using Fisher’s exact test. The analysis revealed a strong statistically significant correlation between the timing of ET and pregnancy outcome (p < 0.0001). D5 ET had a higher statistically significant odd of positive pregnancy outcome (OR = 4.180, 95% CI: 2.410 to 7.174) compared to D3 ET (OR = 0.2393, 95% CI: 0.1394 to 0.4149)
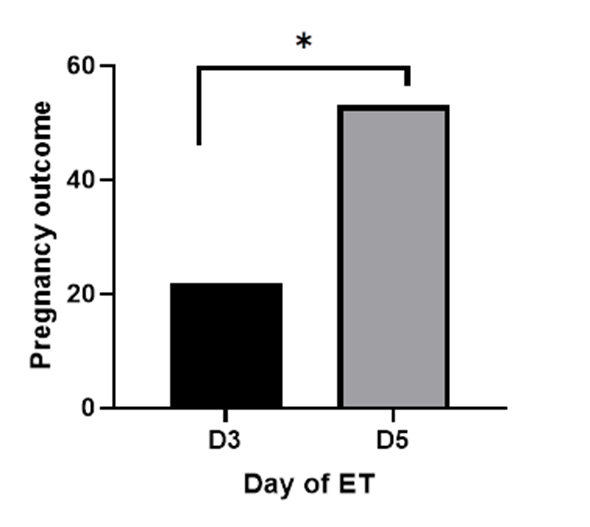
Figure 1: Comparison of the clinical pregnancy outcome between D3 and D5 ET.
* statistically significant difference.
Comparison of clinical pregnancy rates based on the number of embryos transferred.
This study further explores the relationship between the number of embryos (D3 and D5) transferred, the maternal age, and the pregnancy outcome. First, the result (Figure 2) from the logistic regression analysis revealed that the number of D3 embryos transferred, the maternal age and their interplay were not statistically significant predictors of successful pregnancy outcomes. The confidence intervals and odd ratios of these variables, Age (OR = 1.034, 95% CI: 0.7518 - 1.452, p = 0.8403), Number of D3 embryos transferred (OR = 3.130, 95% CI: 0.02988 - 451.5, p = 0.6395), and Age: No of D3 embryos transferred (Interaction Term, OR = 0.9852, 95% CI: 0.8608 - 1.122, p = 0.8237), showed no significant associations. Similarly, the parameter estimates of the same variables revealed no statistically significant associations.
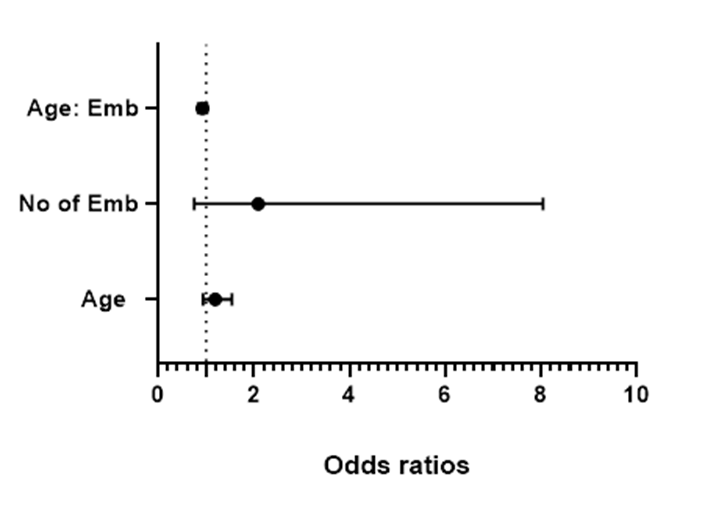
Figure 2: A forest plot diagram showing the odds ratios for the relationship between the maternal age, number of D3 embryos, and pregnancy outcomes.
Figure 3 represent the result of a similar statistical analysis on the impact of the number of day 5 embryo transferred on the pregnancy outcomes, indicating that the maternal age (OR = 1.190, 95% CI: 0.9368 - 1.543, p = 0.1655), number of embryos (OR = 20.94, 95% CI: 0.7529 - 804.1, p = 0.0843) and their interaction (OR = 0.9247, 95% CI: 0.8334 - 1.018, p = 0.1219) were also not statistically significant of predictors of the likelihood of positive pregnancy outcome. A similar outcome was obtained under the parameter estimates, which suggests that the variables do not significantly impact pregnancy outcomes. Figure 4 shows the graphical correlation between the number of embryos (D3/D5) transferred and the pregnancy outcome.
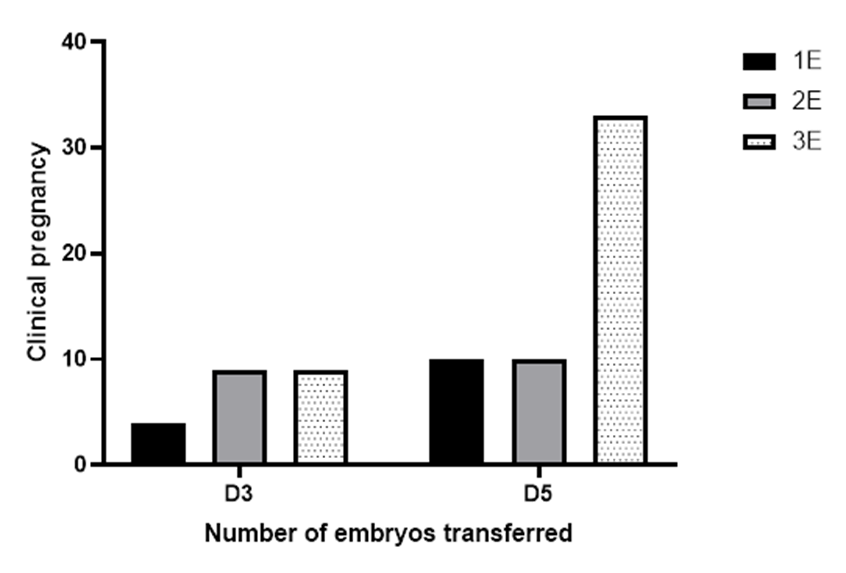
Figure 3: A forest plot diagram showing the odds ratios for the relationship between the maternal age, number of D5 embryos, and pregnancy outcomes.
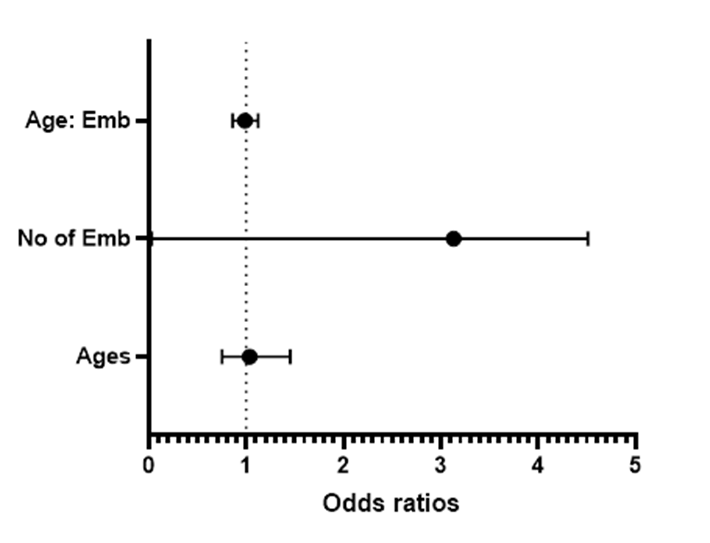
Figure 4: A graphical illustration of the correlation between the number of embryos (D3/D5) transferred and the pregnancy outcome.
Although there is evidence showing that fresh embryo transfer is associated with the risk of ovarian hyperstimulation syndrome (OHSS) when it results in pregnancy [12]. However, some clinics may continue with fresh ET if it is the patient’s preference and there is a low risk of OHSS [13,14].
The benefits of transferring blastocyst-stage embryos versus cleavage-stage embryos remain a controversial topic. Some studies suggest that blastocyst transfer is better, while others report no significant difference in benefit between blastocyst and cleavage-stage embryo transfer [5]. Our findings from this study show that in fresh embryo transfer for patients after ovarian stimulation, the developmental stages of embryos during ET affect the pregnancy outcomes. In this study, the comparison of the clinical pregnancy outcomes between cleavage-stage (D3) and blastocyst-stage (D5) ET shows that D5 fresh ET resulted in a significant clinical pregnancy outcome. This finding aligns with that of the recently published Cochrane review by Glujovsky., et al. This Cochrane review comprising 32 randomized controlled trials (RCTs) reported with moderate-quality evidence that the rate of clinical pregnancy was higher with fresh D5 ET than with fresh D3 ET [4]. Perhaps, this result also supports the claim that blastocysts possess a higher implantation potential than cleavage-stage embryos [15]. It is not uncommon to witness a premature spike in progesterone levels in patients due to ovarian stimulation (regardless of the stimulation protocol) [16,17]. Studies have shown that this premature elevation of progesterone (usually during the follicular phase of ovarian stimulation) negatively impacts the histological architecture and gene expression of the endometrium. This in turn will alter the endometrial receptivity and negatively clinical pregnancy. Nevertheless, there is evidence showing that the transfer of blastocyst may improve the chances of clinical pregnancy [16], which is also seen in our present findings. There is a speculation that extending embryo culture to the blastocyst stage before transfer may be beneficial in counterbalancing the endometrial advancement due to premature spike in progesterone [18,19]. This may be one of the reasons behind the significant difference in the pregnancy outcomes between D3 and D5 embryo transfer we saw in our present investigation.
Interestingly, our investigation suggested that increasing the number of both cleavage-stage and blastocyst-stage embryos transferred does not significantly influence pregnancy outcomes. Ogunsola., et al. in their recent study, gave a similar report that transferring three embryos has no significant advantage over transferring two embryos [20]. However, our findings also suggested that the chances of a clinical pregnancy occurring increase (although not significantly) as the number of embryos transferred increases from one to three. We observed this trend markedly when analyzing the pregnancy outcome with D5 ET and the number of embryos. Furthermore, our findings also aligned with that of Garbhini., et al. which supports that more embryos may be required to be transferred, especially for cleavage-stage embryos, to improve clinical pregnancy in fresh ET [5]. It is essential to note the risk factors associated with multiple pregnancies from multiple embryo transfers. The transfer of more than two embryos presents even higher health risks to the mother and developing fetuses if all embryos are implanted [21,22]. So multiple embryos are transferred to consenting patients who had been informed of the possible risk.
Age is always an important consideration in IVF treatment. Our result here showed that for both groups of patients that receive D3 and D5 embryos respectively, the maternal age was not a significant determining factor for the pregnancy outcome.
It is important to note that the retrospective approach to this analysis serve as a limitation to this study.
To optimize the clinical pregnancy outcome for fresh embryo transfer, it will be beneficial to adopt an extended embryo culture for D5 ET. The transfer of blastocyst-stage embryos to patients on day 5 after oocyte retrieval results in higher pregnancy outcomes compared to cleavage-stage embryos (D3 ET). Although the transfer of multiple embryos should not be encouraged, transferring multiple blastocyst-stage embryos may increase the odds of positive clinical pregnancy outcomes.
We acknowledge the input of the IVF team of nurses for assisting with data collection.
Conception, design and drafting of the manuscript – CWI. All authors (CWI, OEC, IVO, KO, and BO) interpreted results, reviewed the manuscript, and provided substantial advice through the data analysis work, and contributed intellectually to the writing or revising of the manuscript and approval of the final version. All authors read and approved the final manuscript.
The authors did not receive any funding for this study.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
This study obtained ethical approval from St. Ives Specialist Hospital's Institutional Review Board, and all participants provided informed consent with assurances of data anonymization and the voluntary nature of their participation.
Not applicable
The authors declare that they have no competing interests.
Copyright: © 2024 Chibuzor Williams Ifenatuoha., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.