Wilson Charles Wilson*
Tanzania Livestock Research Institute (TALIRI), Uyole Centre, Mbeya, Tanzania
*Corresponding Author: Wilson Charles Wilson, Tanzania Livestock Research Institute (TALIRI), Uyole Centre, Mbeya, Tanzania.
Received: August 27, 2024; Published: September 27, 2024
Citation: Wilson Charles Wilson. “Evaluation of aflatoxin contamination in locally produced chicken feeds in Iringa Municipality, Southern Highlands of Tanzania”. Acta Scientific Veterinary Sciences 6.10 (2024): 36-43.
A study was conducted to assess the aflatoxin contaminations in the locally made feed fed to the Indigenous and improved dualpurpose chickens under semi-intensive and intensive systems in the Southern Highlands of Tanzania. To further understand feed and feeding practices, 101 farming households were surveyed, and then feed samples were collected for laboratory analysis. A total of 32 compounded feed samples were collected and analysed for aflatoxins contamination using AccuScan Gold III reader, a singlestep lateral flow immunochromatographic assay. The two feed ingredients that are most often used to create compounded feed are sunflower seedcake (93%) and maize bran (95%) both of which are very susceptible to aflatoxin contamination. Fish meal (68%), limestone (66%), bone meal (60%), di-calcium phosphate (54%), premixes (46%), and salt (42%) are additional feed ingredients reported by farmers. Only 1% of those surveyed knew what aflatoxin exposure was and how it affected both human and animal health. All feed samples that were evaluated had measurable levels of aflatoxins, and 16% of those samples were above the East Africa Community Standards (> 20 ppb). The highest concentration of aflatoxins was observed in the intensive system and the semiintensive systems with 32.5ppb and 26.6ppb, respectively. Since all feed samples were contaminated, poor storage might increase the level of aflatoxins and threaten the health and production of chicken. The current study’s findings on the contamination of chicken feed with aflatoxins point to significant dangers for human exposure from consuming tainted chicken products. Therefore, we recommend frequent monitoring for aflatoxin contamination along the supply chain as well as capacity building and knowledge dissemination on the proper handling of chicken feed and feed ingredients to reduce contamination.
Keywords: Aflatoxins; Compound Feed; Feed Formulation; Accuscan; Tanzania
Chicken production plays a significant role in supporting the livelihoods in most communities of Tanzania by providing nutrient dense meat and eggs, and income, raised by 86% of livestockkeeping households [1]. Currently, the demand for chicken meat and eggs is already high than the domestic production and supply in Tanzania, and it is projected to increase by three folds by the year 2050 [1,2]. The intensification of chicken industry has been experienced in Tanzania over the past decade involving the transition from subsistence smallholder free-range indigenous breeds to raising large number of improved dual-purpose crossbred chickens and exotic broilers and layers under intensive and semiintensive systems while fed with locally produced feed [3,4].
Feed quality and quantity are major aspects determining the production and productivity of chickens [5,6]. Currently, most poultry farmers in Tanzania rely on locally made feeds and feed materials sourced from their localities i.e. farms, local stores, and markets. The quality of the locally made feeds is often uncertain, and the prices particularly for the protein sources used i.e. fish meal, are costly [7,8]. In Tanzania, quality monitoring of chicken feed and feed ingredients is currently minimal, and processors do not label feed packages displaying the nutrient content information [9]. Furthermore, there is little compliance with recommended feed standards from the Tanzania Bureau of Standards (TBS) due to a lack of awareness, and inadequate extension services [10,11]. Excessive mycotoxins contamination in feeds and feed additives has recently been reported in the country [12-14] posing risks to poultry and human health [15].
Mycotoxins are defined as natural food and feed toxic contaminants produced during fungal growth on crop production and/or storage and transportation [6-8]. Mycotoxins-Aflatoxins of the genus Aspergillus flavus and Aspergillus parasiticus in particular, are the most toxic secondary metabolites of public health concern due to their carcinogenic, mutagenic, hepatotoxic, and teratogenic effect in human and animals [16,17]. The Aflatoxins B1, B2, G1 and G2 are secondary metabolic products produced in food and feed contaminated by Aspergillus species. Aflatoxins residues in animal-sourced food i.e., chicken meat and eggs result from contaminated feed [16] where the ingested aflatoxin B1 is metabolized by chickens into aflatoxin M1 [18,19]. The limits of aflatoxins in food set for human consumption in Tanzania and other East African Community countries is 20 µg/ kg [20,21]. Potential strategies deployed in controlling aflatoxin contamination include application of good agronomic practices and post-harvest handling, the use of biocontrol agents, specifically of Aflasafe [22,23], developing resistant crop varieties, improved regulations, and traceability [20]. Most farmers and consumers in Tanzania as for most developing countries are unaware of aflatoxin contamination and the associated effects to humans and livestock health [15,24]. In Kenya, for example, some consumers were aware of the severe health effects of eating mould-contaminated food but were uniformed of the risks of consuming products from animals fed contaminated feed [25]. The current study aimed to assess the aflatoxin contaminations in the locally made feed fed to the Indigenous and improved dual-purpose chickens raised under semi-intensive and intensive systems in the Southern Highlands of Tanzania.
The research was carried out in Iringa municipality, Southern Highlands of Tanzania where chickens are mostly raised under semi-intensive and intensive chicken production systems [4]. The municipality was purposively selected due to its potential for commercial chicken production and the presence of hatcheries, grain millers, and feed processors established over the last decade.
A cross-sectional survey was executed in the Iringa Municipality from November to December 2020 whereby 101 farmers rearing dual-purpose chickens were interviewed (Table 1) using a semistructured questionnaire designed using Open Data Kit (ODK) software (https://opendatakit.org/). We conducted a stratified sampling of farms in fourteen Wards based on the relative number of households keeping chicken according to the extension office statistics. The interviews were conducted by trained enumerators where adult respondents involved in chicken management were interviewed from two pre-defined rearing systems i.e., semiintensive and intensive systems. The rearing systems were primarily defined based on housing and feeding systems [4] whereby 81% and 19% of the respondents raised chickens in semiintensive systems, respectively.
The survey questions included the awareness of farmers on aflatoxin contamination and its effects on animal and human health, management practices in chicken production, the common types of feed and feed ingredients used in formulating locally feed, sources of feed ingredients, and their availability, prices of feed and feed ingredients, and the quantities and prices of the products produced and marketed as further elaborated in our recent study [21]. The current study focuses on the findings on feed safety aspects of aflatoxin contamination in the locally made feed fed to the Indigenous and improved dual-purpose chickens raised under semi-intensive and intensive systems as further explained below.
A total of 32 feed samples were collected for Aflatoxin assessment (one sample from each farm, sampled from the entire mixed ration), with 23 and 9 samples from semi-intensive and intensive farms, respectively (Table 2). 1000g of the representative feed sample were collected from the respective farms and packed into a labelled clean plastic fridge bag, and subsequently transported to the International Institute of Tropical Agriculture (IITA) pathology laboratory in Dar es Salaam, Tanzania using a cooling box on weekly basis. At IITA laboratory, all samples for mycotoxin analysis were stored in refrigerator at -4°C before analysis. The samples were analysed for aflatoxin contamination using an AccuScan Gold III reader, a single-step lateral flow immunochromatographic assay and further compared with the recommended standards from the Tanzania Bureau of Standard and the East Africa Community Standards [21,26].
The quantitative data resulting from the interviews were tested for normality and analyzed using R Statistics to obtain descriptive statistics of the studied population. The Generalized Linear Model (GLM) was used to test the effects of the rearing system (semiintensive and intensive), breed (indigenous and improved crossbred) and interactions at a 5% level of significance on flock size, total number of laying hens in the flock, egg production and weekly lay (%) per flock per week using the following statistical model:
Yijk = μ + Ri +Bj + Ri × Bj+ eijk
Where;
Yijk = an observation for a given variable
μ = overall mean
Ri = effects of the ith rearing system (1=semi-intensive, 2=intensive)
Bj = effect of jth breed (1=indigenous, 2=improved cross bred)
Ri × Bj = the interaction between the rearing system (i) and breed (j)
eij = residual error term
The R package 4.2.1 was used to investigate the effect of the rearing system and breed on the level of aflatoxin contamination. The aflatoxin content of the feed per farm was plotted against the recommended values using the ggplot2 package in R. The graphs illustrating the level of aflatoxins and moisture content of the feed were drawn, and compared to the recommended limits, (<20 ppb).
The average flock size in the municipality was 100 chickens per household without significant differences between breed and rearing system (Table 1). Of the 101 interviewed farms, 64% raised indigenous breeds while 36% were keeping the improved crossbred chickens across both systems. The total number of laying hens in the flock was influenced by the breed of chickens and production system (P < 0.05). Furthermore, the households (P < 0.05) (Table 1). The rearing system and breed of chickens keeping the improved breeds of chicken under the intensive system significantly affects the number of eggs and weekly percentage lay had more laying hens than households keeping indigenous breeds in the flock (P < 0.05).
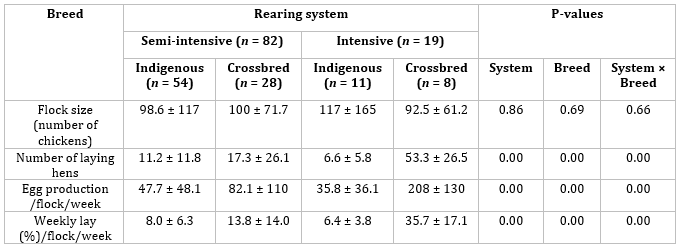
Table 1: Chicken farming typology in the Iringa municipality (mean and standard deviation).
Based on the household survey, the findings shows that all farmers interviewed fed their chickens with the locally processed feed where the most common feed ingredients used in formulating chicken feeds included maize bran (95%) and sunflower seedcake (93%). Other feed ingredients reported by farmers included fish meal (68%), limestone (66%), bone meal (60%), di-calcium phosphate (54%), premixes (46%), salt (42%), sorghum (29%), soybean meal (16%), maize meal (11%), rice polishing (10%), blood meal (10%), lysine (2%), and methionine (2%).
The findings from the survey shows that only 1% of the respondents interviewed were aware of aflatoxin contamination and its effects on animal and human health. The laboratory analysis shows that all feed samples tested positive for aflatoxins, with 16% exceeding the East Africa Community/TBS limits (20 g/kg). The highest concentrations of aflatoxins were found in feed samples collected from farms raising indigenous chickens in both semiintensive and intensive systems, with 32.5 g/kg and 26.6 g/kg, respectively (Figure 1 and Table 2). In the semi-intensive system, 12% of the collected samples showed an excess moisture content, while most farms have low moisture content in the feed, implying less risk of increasing the level of aflatoxins.
All farms with higher levels of aflatoxins (>20 µg/kg) had a low weekly percentage lay although there was no statistically significant difference due to the small sample size (Figure 2).
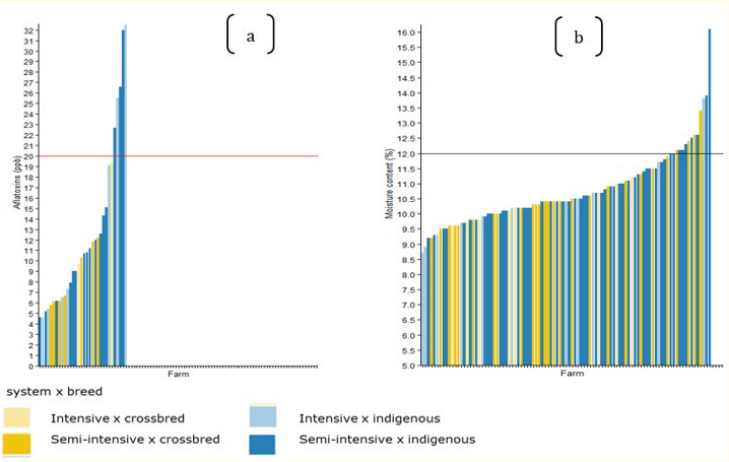
Figure 1: (a) Aflatoxin and (b) moisture content assessment in relation to the maximum limits (horizontal lines) set by the East African Standards (20 parts per billion (ppb) and 12%, respectively.
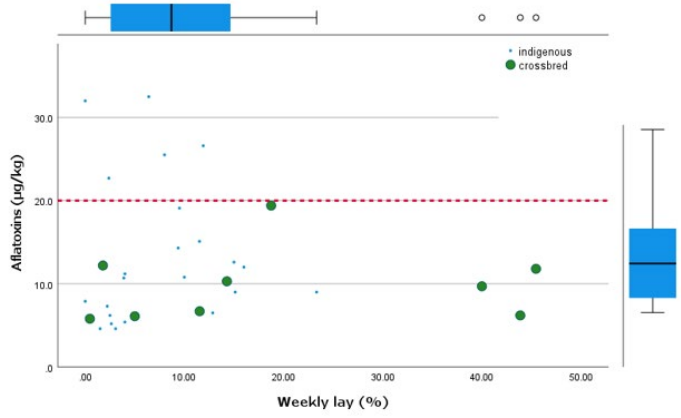
Figure 2: Relationship between the aflatoxin contamination and weekly percentage lay. The red dotted line shows the maximum East Africa Community standards (>20 µg/kg).
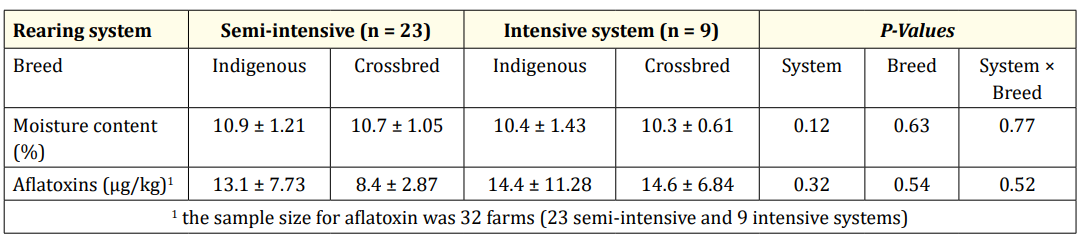
Table 2: Nutritional quantity and quality of chicken feed in the study area (Mean and standard deviation values and P-values).
In this research, we set out to assess the level of aflatoxin contaminations in the locally made feed fed to the Indigenous and improved dual-purpose chickens raised under semi-intensive and intensive systems in the Southern Highlands of Tanzania. The study reveals that the intensification of chicken industry in Tanzania involves the transition from keeping free-range chickens to medium-sized smallholder farming systems keeping chickens under intensive and semi-intensive systems where chickens are fed with the locally formulated feed. Similar findings have been reported in the country, where sub-divisions of chicken farming systems resulted into five farm types i.e. small-scale free-range, small-scale semi-intensive, medium-scale semi-intensive, smallscale intensive and medium-large scale intensive farms [4]. Moreover, despite having large flock size and number of laying hens under the intensive system, the utilization of commercial feed chicken feed is low. Commercial chicken feeds are costly and less affordable by most smallholder farmers in Tanzania, often sold in large quantities of 50 kg bags [5]. Considering the costs of commercial feed, some farmers keeping chickens under intensive and semi-intensive system partly fed their chicks with commercial starter feed during the early stages of growth and ultimately switch to locally formulated feed to reduce the cost of production [5,27].
The common feed ingredients used by farmers in the study area i.e. maize bran and sunflower seedcake are grown within the region [21,28]. Those feed ingredients are most prone to aflatoxin contamination [29]. High aflatoxin contamination may lead to decreased chicken production and productivity due to depressed feed intake, laying performance, egg quality and hatchability and ultimately lead to deposition of aflatoxin residues in eggs and meat [30,31] The current study findings show that all feed samples collected from the study area contains detectable amount of aflatoxin contamination The high level of aflatoxin contamination is linked to low knowledge and awareness of farmers on its presence, associated health impacts, and control strategies as reported by 99% of the respondents interviewed in the present study. Moreover, the present study reveals that the level of aflatoxin contamination is particularly high in the households keeping indigenous chickens where they exceeded the maximum aflatoxin limits (>20 g/kg) in both systems, posing severe threats to chicken production and human health. Despite the low moisture content observed in feed samples from most farms, there is a significant potential rise in aflatoxin contamination due to farmers’ lack of knowledge about the existence, effects, and control of aflatoxins [29]. According to research, aflatoxins have a negative impact on laying performance, feed intake, eggshell thickness, and egg hatchability, as well as the deposition of aflatoxin residues in eggs and meat [32-34].
The current study’s findings show no significant link between aflatoxin concentration and egg production (% weekly lay), which could be attributed to the limited sample size. According to research, free-range chickens are more susceptible to aflatoxin contamination than semi-free-range chickens because they rely on scavenging and are exposed to contaminated feed [35]. As a result, because most Tanzanians prefer products from indigenous freerange chickens, interventions in chicken management and feed quality control are needed to reduce aflatoxin residues in chicken meat and eggs [2,36].
As noted in recent Tanzanian studies, aflatoxin contamination in chicken feed and feed materials implies a substantial risk of food contamination [37-39]. The Tanzania Bureau of Standards and the Tanzania Veterinary Laboratory Agency are currently in charge of food and feed quality and safety requirements. Unfortunately, the latter organisation lacks equipment for mycotoxin feed safety analysis and relies on outsourcing from other institutions elsewhere [40]. The equipment outsourcing costs are high, which raises the costs of feed analysis for farmers and feed processors and so might lead to skipping mycotoxin analysis to evade the costs and might increase risks of aflatoxin contamination, threatening animal, and human health [41].
The intensification of chicken industry in Tanzania requires improved access to quality and safe feed. The presence of detectable aflatoxins in all feed samples analysed in the present study indicates high risks of aflatoxin contamination in chicken feed and aflatoxin residues in meat and eggs. Lack of farmers’ knowledge and awareness about the existence, effects, and control measures of aflatoxins might increase the level of aflatoxins contamination, threatening chicken health and production. The presence of aflatoxin contamination in chicken feed indicates significant risk of human exposure from eating contaminated chicken products. To reduce contamination, we recommend periodic monitoring for aflatoxin contamination across the supply chain, as well as capacity building and knowledge dissemination on the existence, effects, and control measures of aflatoxins.
We gratefully acknowledge NWO-WOTRO’s financial support for “The Missing Middle project: Food system transformation pathways to link actions at multiple levels to Sustainable Development Goals (SDGs), particularly SDGs 2, 12, 13, and 15 in Tanzania and Vietnam” (Grant number W07.303.109) and the Netherlands-CGIAR Scientific Expert Programme. We highly appreciate the support from the municipal livestock extension office and participating farming households for their time and willingness to provide data. Mr Edson Florence provided invaluable assistance during fieldwork planning, data collecting, and laboratory analysis. We appreciate the technical advice and assistance provided by Dr. George Mahuku, Mr. Jackob Njela, and Hilary from the IITA pathology laboratory. We would like to express our gratitude to the Tanzania Veterinary Laboratory Association (TVLA), particularly Ms Wende Maulaga, for supporting laboratory analysis of the nutritional quality of the feed as well as technical guidance on sample collection and handling. We would like to thank Eva Thuijsman and Thomas Delaune for their help in generating R scripts, as well as Prof. Rene Kwakkel for his advice on the study design.
Copyright: © 2024 Wilson Charles Wilson. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.