Ruiz Castañeda Martin1, Ruiz Tovar Diana Isela2, Lara González Cesar3, Galán Madrigal Armando3, Luna Cortés Alejandro3, López González Hilda Eugenia3, Rodríguez Flores José Reyes4, Grageola Núñez Fernando5, Mireles Flores Salvador1*
1Department of Animal Production of the University Center of Biological and Agricultural Sciences of the University of Guadalajara
2Intern of the Veterinary Medicine Career of the University Center of Biological and Agricultural Sciences of the University of Guadalajara
3Department of Animal Production, Quality Control and Research and Development of BIOO ZOO Laboratory
4Department of Veterinary Medicine of the University Center of Biological and Agricultural Sciences of the University of Guadalajara
5Academic Unit of Veterinary Medicine and Zootechnics, Autonomous University of Nayarit, Mexico
*Corresponding Author: Mireles Flores Salvador, Department of Animal Production, Mexico.
Received: July 18, 2024; Published: August 31, 2024
Citation: Mireles Flores Salvador., et al. “Evaluation of Neutralizing Antibody Titers against Rabies in Cattle Vaccinated with SAD Strain, Modified Active Virus". Acta Scientific Veterinary Sciences 6.9 (2024): 58-63.
With the objective of evaluating the titers of neutralizing antibodies against rabies in cattle vaccinated with the modified active virus SAD strain, a study was carried out in an extensive livestock system with continuous grazing ("Rancho Jaltitan"), municipality of Ixtlahuacán del Río, Jalisco, Mexico., where twenty-six calves of both sexes were used, between six and eleven months old, cross between Limousin and Charolais, clinically healthy and with antibody titers of less than 0.6 IU/mL against bovine paralytic rabies (BPR). A completely randomized design was carried out, where the animals were divided into two groups under the same housing and feeding conditions, one was a control and the other was administered the investigational vaccine on day. Blood samples were collected on days 7, 14, 21 and 45 after vaccination. Antibodies were determined with the rapid fluorescent focus inhibition test. The data obtained were processed with analysis of variance and the difference between means (P<0.05) was evaluated according to the Tukey method. It is observed that on day seven post-vaccination the treatment with SAD strain showed antibody levels of 0.44 IU/mL, on day 14 =2.22 IU/mL, on day 21 = 2.97 IU/mL and 3.53 IU/mL on day 45. Control group, antibody levels lower than 0.6 IU/mL were observed throughout the study. It is concluded that the SAD strain rabies vaccine adequately stimulated the seroconversion of vaccine antibodies against paralytic rabies in cattle from day 14 onwards.
Keywords: Paralytic Rabies; SAD; Bovine; Seroconversion; Vaccine
The Rabies is an infectious, acute and fatal disease, which has a high tropism for the central nervous system, caused by the rabies virus, one of the 16 viruses that make up the Lyssavirus genus of the Rhabdoviridae family, it has a worldwide distribution, It is mandatory to notify [11,18] and varies between 25 days and 5 months in the case of cattle [24,31]. It can affect any warm-blooded animal, except birds [5]. It is transmitted to man or animals mainly through the saliva of a sick animal or contaminated material [17], such as the consumption of infected corpses [2,16] and there are even reports of cases in humans who have acquired the disease when exposed., to aerosols [13,30].
In the American continent, blood-sucking bats are the main source of human rabies cases [19]. In tropical and subtropical areas, the rabies transmitted by vampire bats is also one of the main causes of livestock mortality, affecting both subsistence and commercial farmers throughout the distribution area of this bat (from Argentina and Uruguay to northern Mexico) [19], even in regions that for decades had been considered free of this disease, attacks on livestock by hematophagous bats of the genus Desmodus rotundus (D. rotundus) have occurred more frequently [5].
Bovine paralytic rabies (RPB) or derriengue is considered an epidemic and recurrent disease caused by the rabies virus transmitted by the vampire D. rotundus, which mainly affects cattle, horses, and less frequently other domestic species such as sheep, goats, pigs, some wild animals and man [6], in cattle it is usually characterized by paralysis of the posterior train [5].
In Mexico, according to reports from the SENASICA zoosanitary campaigns directorate, currently RPB is considered endemic and is distributed from the state of Sonora to the state of Chiapas and along the coast of the Gulf of Mexico in Tamaulipas to the Yucatan peninsula. (twenty-one). Despite the strategies implemented by authorities and ranchers such as vaccination, capture of bats, among other activities to control and reduce RPB, during the year 2020, 274 were reported with distribution in 20 states of the country, where Veracruz is the state that reported highest number of positive cases with 52; Veracruz being an entity with a warm- humid climate with an average annual temperature of 23°C and relative humidity of 75%. These factors are ideal for the blood-sucking bat (D. rotundus) to adapt and reproduce, which may affect the incidence of rabies in this area. After Veracruz, the important reporting states are Puebla [35], Nayarit [31], San Luis Potosí [25], Hidalgo [23] and Chiapas [19,21].
According to reports from the SENASICA zoosanitary campaigns directorate, RPB is currently considered endemic in Mexico. It is distributed from southern Sonora to Chiapas and along the coast of the Gulf of Mexico from southern Tamaulipas to the Yucatan Peninsula [21]. Despite the strategies implemented by authorities and ranchers such as vaccination, bat capture, among others, to control and reduce RPB. According to the data reported by SENASICA, 364 cases were reported during 2017; 2018, 423; 2019, 239; 2020, 229 and in 2021, 282 cases. The states with the highest incidence are Veracruz, San Luis Potosi, Chiapas, Hidalgo, Nayarit and Puebla [21].
The control of bat (D. rotundus) populations is through anticoagulants and has been shown to be a temporary measure of long-term spread and progression of the disease [1,3,14,26]. Among the control strategies for RPB, there is pre-exposure vaccination, which is an efficient and profitable action to reduce the incidence of the disease. In this sense, over time we have worked to produce anti-rabies vaccines that are safe and effective.
There are different vaccine strains against rabies, which allow the formulation of inactive and attenuated vaccines; the first with dead virus; while the latter are formulated from a modified live virus, which replicates in the host and leads to the formation of antibodies by simulating an infection [20,28]. Among the rabies vaccines that use modified live viruses, we find that many of these are derived from Evelyn Rokitnicki Abelseth (ERA), which emerged in 1960 after passage through several lines, and that in turn this comes from the original Street Alabana strain. Dufferin (SAD) that was isolated from a rabid dog in 1935, eventually the ERA strain was transferred to the Centers for Disease Control and Prevention (CDC), and in 1970, after several passages through the line cell BHK-21 (derived from Syrian hamster kidney), the SAD Bern strain arises, which is considered the origin of all current vaccine SAD strains; Among these, the SAD B19 strain was selected for its thermostability and low pathogenicity [8,12,20].
Currently, bovine vaccines are formulated mainly with attenuated virus and some companies inactivate them; The serological response with satisfactory effect is described, but there are factors that determine the amount of antibody, such as the age of vaccination; Before 6 months, maternal antibodies can interfere with the immune response. Another situation is the number of doses and boosters that will be applied [34].
In a study, post-vaccine antibody levels were determined in cattle immunized with a commercial product that includes the SAD strain, which is capable of triggering a protective immune response [22].
Despite the efforts made through vaccination and the capture of D. rotundus, officially, significantly more cases of RPB continue to be reported. On the other hand, the continuous supply of different vaccine strains from non-certified laboratories generates uncertainty among livestock farmers. On the efficacy and safety of vaccines, for this reason, the present research study proposes to demonstrate that a commercial vaccine formulated in Mexico with the SAD strain is capable of producing protective antibody titers in cattle (equal or greater than 0.6 IU/ml), according to what is indicated in NOM-067-ZOO-2007.
We conducted a study for 89 days in an extensive livestock system with continuous grazing in the “Rancho Jaltitan” of the municipality of Ixtlahuacán del Río, Jalisco, Mexico located on the Guadalajara-Saltillo highway at km. 60 with a north latitude of 200 97' west longitude 1030 22' and an altitude of 1,700 meters above sea level. The average annual temperature ranges between 20 to 22 0C, the direction of the winds is very variable and the average annual rainfall is 800 mm³. The climate is considered semi-dry and semi-humid according to the Köppen-Geiger classification. climates of the world. 26 earring calves were used for identification; these animals and their mothers had not been previously vaccinated against RV (rabies virus). The calves were of both sexes, between six and eleven months old, a cross between Limousin and Charolais, clinically healthy, which were bled 41 days before vaccination in order to verify that they had non-protective antibody levels against RV, being average antibody titers less than 0.6 IU/ml determined through the rapid fluorescent focus inhibition test (RIFFT) [23], a method described by the World Organization for Animal Health (WHO).
For the study, a completely randomized design (DCA) was carried out, where the animals were divided into two groups; control (Group B) being n=13 (7 males and 6 females) and the experimental group (Group A); n=13 (5 males and 8 females). To which a lyophilized vaccine containing active modified SAD strain rabies virus obtained in tissue culture with a minimum of 1x105.1 DLR/Dose was administered (Table 1). Housing and feeding conditions were the same for both groups throughout the study. Blood samples were collected from each animal from the coccygeal vein on days 7, 14, 21 and 45 after vaccination to obtain the serum and determine the neutralizing antibody titers in each case using the RIFFT test according to the terrestrial manual of WOAH (2018) used a canine reference serum standardized at a potency of 2 IU/mL [32].
A comparative descriptive study was carried out and an analysis of variance (ANOVA) was performed on the difference in means of the data, using the STATGRAPHICS Centurion IV program (25). This study was evaluated and approved by the internal council for the care and use of laboratory animals (CICUAL), of BIO ZOO S.A de C.V.
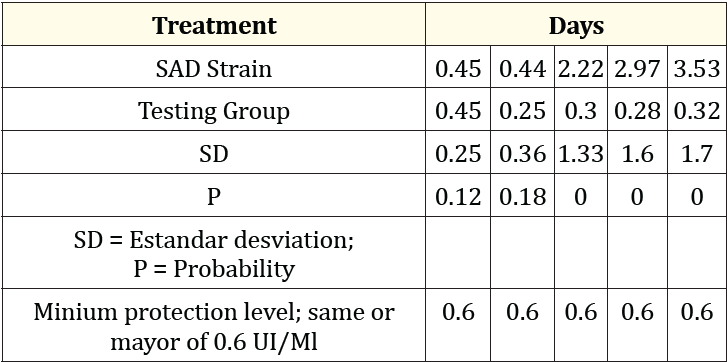
Table 1: Results of the administration of the lyophilized vaccine with the active modified rabies virus strain SAD (Group A).
Graph 1 shows the average antibody titers (IU/ml) over time against rabies in cattle. Titration below the protective titers of 0.6 IU/ml (P > 0.05) was observed on day 41, where 11.54% (3 animals) exceeded the minimum level of protection. These values 0.80, 0.85 and 1.00 IU/ml. The rest of the animals (88.5%) were found below the minimum neutralizing levels against RPB according to NOM-067 ZOO-2007.
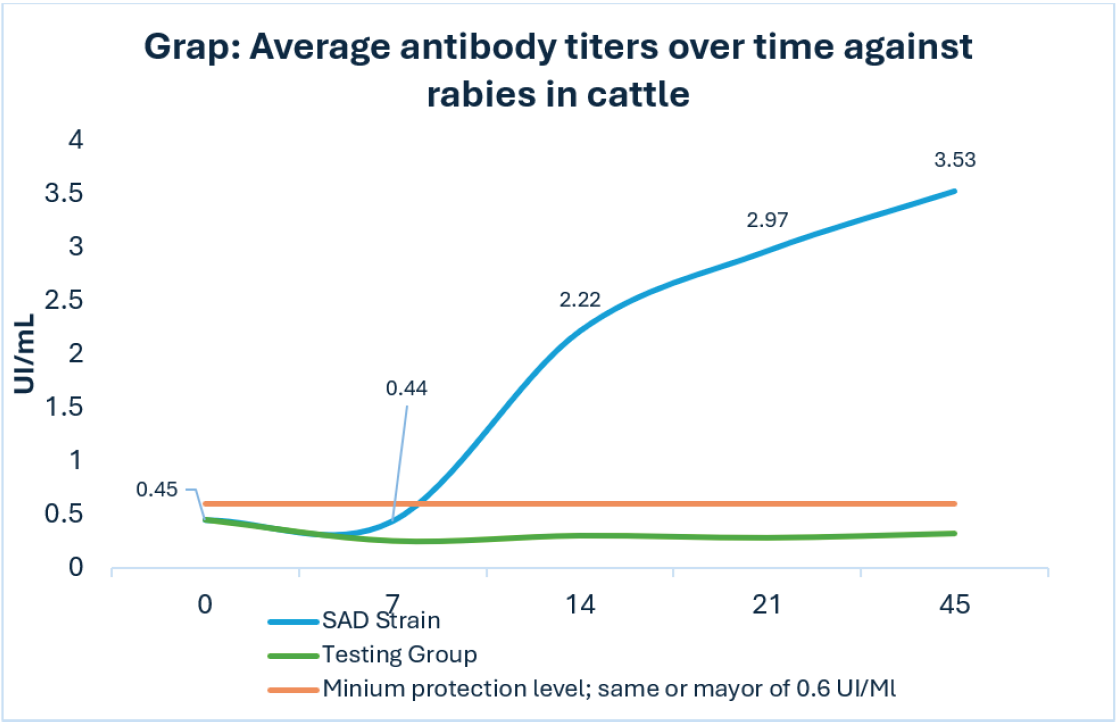
Graph 1: Average antibody titers over time against rabies in cattle.
The presence of protective titers of antibodies against rabies in 11.54% of the population prior to vaccination in the study population coincide with what was described by Gilbert., et al., [33], who mentions that the presence of neutralizing antibodies in the 12% of the bovine herd, prior to vaccination. Ellison., et al., [7] demonstrated the presence of rabies neutralizing antibodies (7% of 398 sera taken) from unvaccinated bovines. Likewise, with Gilbert., et al., [9] who reported that in Peru, 11% (7 of 63) of neutralizing antibodies to the rabies virus were registered in humans. Data suggesting non-fatal exposure to rabies virus and associated with predation by blood- sucking bats. The antibody titers found prior to vaccination coincide with what Gold., et al., [11] describe, where the presence of specific antibodies was recorded in humans, domestic animals and wild animals without vaccination and apparently healthy, this is possibly due to the prior exposure to the antigen and challenge to the virus in the field through direct contact with an animal that acts as a reservoir of the virus; however, it is very uncertain to determine the time, intensity or frequency of the possible infection that generated these antibodies [22].
The vaccine was administered on day zero of the study. On day 7 after vaccination, treatment with the SAD strain showed average antibody titers of 0.44 IU/ml (P > 0.05); However, only 15% (2/13) of vaccinated animals showed adequate protection (greater than or equal to 0.6 IU/ml according to NOM-067 ZOO 2007). On day 14, an average seroconversion of 2.2 IU IU/ml (P < 0.05) was observed, where 84.6% (11/13) were found with adequate protection. On days 21 and 45, averages of 2.97 IU/ml and 3.53 IU/ml were observed (P < 0.05). respectively with 100% of the protection protected (13/13) in both cases. The control group had antibody levels lower than 0.6 IU/ml throughout the study.
Only 15.0% of the animals in group A began to titrate antibodies on day seven, and on day 14 it was observed that the majority of the study population are from Group A (84.6%) showed an adequate level of seroconversion and with an average of 2.22 IU/ml of antibody titers (Graph 1), both results higher than those reported by Quiñonez [22], who reports a seroconversion of 60.0% of the animals with an average of 1.39 IU/ml, on day 15 post vaccination with the SAD strain.
Starting on day 21, one hundred percent of the population in the study of group A (SAD strain) was protected and continued to increase until day 45, with average antibody titers of 2.96 and 3.52 IU/ml respectively. (Graph 1), these results were higher than what was mentioned by Quiñonez [22], who reported 1.97 and 2.9 IU/ml respectively after the administration of the vaccine.
In this study, a dose of the SAD strain modified live virus rabies vaccine administered intramuscularly in cattle was shown to increase the antibody titer to 3.52 IU/ml at 45 days, exceeding that reported by Lui et. to the [15]. When they evaluated the immunological efficacy of inactivated vaccines against rabies indicated for canines, in said study it was observed that the administration of a dose at 90 days in beef, dairy and camel cattle reached a titer of 0.93, 0.84 and 0.68 IU/ml respectively, then decreased until the titration disappeared after 180 days. Wangmo et. to the [29], measured the antibody titer of an anti-rabies vaccine with inactivated virus (Raksharab, Indian immunologicals) when it was applied intramuscularly in cattle and where lower titers were obtained than those found in this study (day 14: between 0.75 to 1.0 and on day 30: between 1.0 to 1.25 IU/ml).
Currently, there are few studies that have described the behavior of rabies vaccines in cattle; and according to the national panorama of bovine paralytic rabies in Mexico issued by SENASICA, it is mentioned that the increase in RPB cases in Mexico, among other factors, could be due to insufficient vaccination coverage, which favors the presence of a significant number of susceptible animals in areas of high viral transmission [27]. Having mentioned the above, strategies that include training sessions for rural livestock communities at risk must be strengthened and efforts redoubled in the implementation of vaccination campaigns against RPB, as well as encouraging the monitoring of vaccinated animals.
It is concluded that the modified live virus vaccine against rabies in cattle with the classic SAD strain adequately stimulated the seroconversion of vaccine antibodies against paralytic rabies in cattle from day 14 onwards. Since rabies is a zoonotic disease, it is recommended to carry out other investigations to contrast this study.
Copyright: © 2024 Mireles Flores Salvador., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.