Anand Laxmi Nidamanuri1*, Shanmugam Murugesan1, Jayakumar Sivalingam2, Rama Krishna Mahapatra1 and Sri RajaRavindra Konadaka2
1Animal Nutrition, Health and Physiology, Directorate of Poultry Research,Rajendranagar, Hyderabad, Telangana, India
2Animal Genetics and Breeding, Directorate of Poultry Research, Rajendranagar, Hyderabad, Telangana, India
*Corresponding Author: Anand Laxmi Nidamanuri, Principal Scientist, Animal Nutrition, Health and Physiology, Directorate of Poultry Research, Rajendranagar, Hyderabad, Telangana, India.
Received: August 07, 2024; Published: August 27, 2024
Citation: Anand Laxmi Nidamanuri. “Plasma Hormone Amino Acid Expression of Amino Acid Transporters Hormone Receptors and their Modulation by Organic Selenium in Laying Hens”. Acta Scientific Veterinary Sciences 6.9 (2024): 33-45.
The objective was to study the differential level of physiological parameters during the laying period and efficacy of organic selenium in modulating the parameters and their relationship with egg production in Aseel breed. Analysis of plasma levels of amino acids by LCMS, melatonin, ghrelin, estrogen, and progesterone by enzymeimmunoassay and the expression of receptors and amino acid transporters by q PCR technique were conducted for the early (EP) and middle (MP) laying periods. Two groups, one being a control group, were reared on a basal diet containing 0 mg of organic Se, but the treatment group diet was supplemented with 0.2 ppm organic Seleno yeast (SY). Compared with the control group, the treatment group had a lower mean level of ghrelin hormone (GHL) and a greater level of steroid hormones at the MP timepoint than at the other timepoints. Supplementation with SY decreased the levels of all hormones except estradiol at the EP and increased the plasma levels of all four hormones at the MP stage. Whereas levels of plasma essential amino acids decreased at both time points. Further the relation between the expression of melatonin receptors (MNTRs) in the jejunum to the hormone concentration at both the EP and the MP stages, and the expression of the GHL receptor (GHLR) to the GHL concentration in both the tissues at EP and MP was inversely proportional. The expression of amino acid transporters decreased in the jejunum and increased in magnum tissue at EP followed by increased trend at MP. The levels of GHL, steroid hormones and plasma amino acids vary between the early and mid-periods of lay and they can be suitably modulated by supplementation with SY, which significantly increases egg production at both the EP (3%) and MP (5%) stages.
Keywords: Aseel; Endocrine; Feed Additive; Gene Expression; Laying Period
SY: Seleno Yeast; CG: Control Group; TG: Treatment Group; EP: Early Laying Period; MP: Mid Laying Period; rpm: Revolutions Per Minute; oC: Degree Celsius; SEM: Standard Error of the Mean; MET: Melatonin; EST: Estradiol; PROG: Progesterone; GHL: Ghrelin; ELISA: Enzyme Linked Immunosorbent Assay; DEPC: Diethyl Pyrocarbonated; DNA: Deoxyribonucleic Acid; RNA: Ribonucleic Acid; aats: Amino Acid Transporters; MNTR: Melatonin Receptor; GHRL: Ghrelin Receptor; Standard abbreviations have been used for 19 amino acids and four amino acid transporters
Aseel is an important native breed of poultry in India that has fighting characteristics, high stamina, delicious meat and can thrive under harsh conditions [1-3]. Usually, Aseel birds lay eggs at approximately 27-29 weeks of age, and the annual egg production is 60-80 eggs [4,5]. Because of its low egg production, it is a less preferred breed [6].
The gastrointestinal mucosa is the largest source of melatonin and may also influence other hormones [7,8]. The action of melatonin is independent of photoperiod [9,10]. Various functions have been attributed to melatonin, including reproduction [11]. Ghrelin regulates the levels of steroid hormones in pigs [12,13]. The plasma concentrations of estrogen and progesterone vary according to age in this breed according to ovulation requirements [14].
Amino acids play an important role in the formation of proteins in the body [15,16]. They are further utilized for growth, enzyme synthesis and egg production. The digestibility of amino acids is affected by changes in the nutrient composition of the diet [17].
Compared to the egg production percentage in inorganic supplemented poultry groups, the groups supplemented with organic seleno yeast (SY, 0.3 ppm) had enhanced production [18]. Furthermore, supplementing sodium selenite at 0.3 ppm resulted in egg production that was similar to the control. However, most of these studies were conducted under stressful conditions. Providing aged hens with SY supplementation at 70 weeks of age increased only egg weight and albumen quality [19]. Similarly, reports on the effects of organic Se on 30- to 50-week-old breeder hens and on laying hens have been published [20-22].
Organic selenium affects egg parameters and the available reports are contradictory. The present study investigated the effects of the addition of an additional 0.2 ppm of SY in the basal diet, on the physiological parameters and production of the Aseel during the laying period (26-36 weeks of age). To date, no work has been carried out with respect to endocrine and molecular parameters and their modulation by SY.
Work was conducted in accordance with ethical standards and approved by the Ethics and Biosafety Committee of the institution. Research on animals was conducted according to the institutional committee on animal use (IAEC/DPR/19/3) Regn. No. 355/GO/ RBi/S/01/CPCSEA.
One hundred Aseel pullets with uniform body weights (1440 ± 11 g) at approximately 24 weeks of age were selected from our institute farm situated in Hyderabad, Telangana (18.11°N latitude and 79.01°E longitude), India. The birds were acclimated to basal feed consisting of maize and soybean (Table 1) for 2 weeks. Water was provided ad libitum. The birds were distributed into individual California-type cages and housed in an open side housing system. At the beginning of the 26-week period, the hens were divided into two groups with equal numbers of hens. Each group included 50 birds and had ten replicates, with five birds in each replicate. The control group (CG) was provided basal feed (Table 1), and the treatment group (TG) was provided basal feed supplemented with 0.2 ppm organic selenium. Selenium-enriched yeast (SY, Seleno SourceTM AF 2000), a commercial organic product purchased from Nurture Organics, Karol Bagh, New Delhi, was added to the basal feed of the treatment group. All other components of the basal feed were supplemented according to the standard requirements at our institute for layer hens under a 16 h light:8 h dark cycle. The temperature ranged between 27 and 30 °C, and the relative humidity ranged from 65-70% during the course of the experiment. Under standard conditions, feed was provided at 110 g/bird/day. For the present experiment, experimental tenure was divided into two stages, i.e., from 26-30 weeks (early laying period, EP) and then from 32-36 weeks of age (mid-laying period, MP).
Blood samples were collected from the wing vein of ten birds from each group at random in heparin-coated tubes for estimation of melatonin, ghrelin, estradiol, progesterone hormones and plasma amino acids. Blood was collected from the same ten birds at weekly intervals.
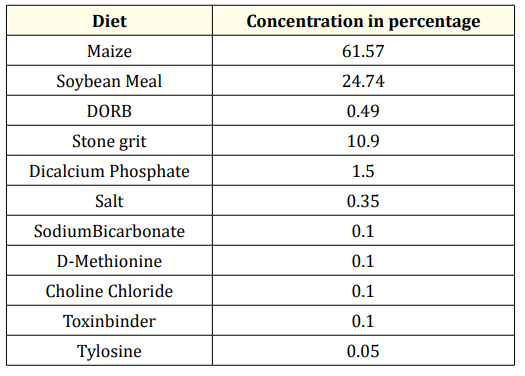
Table 1: Composition of the Basal Diet.
Estimated values
ME-2625Kcal; CP- 17%, LYS-0.84%, MET-0.33%, Ca-3.9%, Available P-0.36%,, Na-0.18%
Analysed values: CP-16.5%*, Ca-3.5%.
Nutrient composition
Provided (mg/kg diet): thiamin 1; pyridoxine, 2; cyanocobalamine, 0.01; niacin, 15; pantothenic acid, 10; a tocopherol, 10; riboflavin, 10; biotin, 0.08; menadione, 2; retinol acetate, 2.75; cholecalciferol, 0.06; choline, 650; copper, 8; iron, 45; manganese, 80; zinc, 60; inorganic selenium, 0.30; hydrated sodium calcium alumino silicates, 800; phytase, 375 units. Treatment group (TG) - Basal feed + 0.2ppm Yeast enriched selenium.
* As fed basis.
After blood samples were collected, the tubes were kept on ice and transported to the laboratory. The samples were further centrifuged at 3000 rpm for 15 min. The plasma obtained as the supernatant was stored at -20 °C for analysis of amino acids and hormones.
The blood samples collected at weekly intervals were analyzed for different hormones. The mean ± SE values for each hormone in each bird were calculated for each week, and the mean ± SEM was subsequently calculated for the EP and MP stages. The plasma levels of the hormones melatonin (MET, E12M0005), estradiol (EST, E12E0023), and progesterone (PROG, E12P0200) were assayed using commercial chicken ELISA kits (BlueGene Biotech, Shanghai).
Ghrelin (GHL, BC-ECh 040174) was also measured using a commercial chicken ELISA kit (Biocodon Technologies, Kansas, USA) according to the manufacturer’s instructions. Briefly, ELISA kits for the first three hormones were used for the competitive enzyme immunoassay technique utilizing the respective anti-antibody and HRP conjugates. After the final reaction, the absorbance of the color that developed in the samples was measured at 450 nm in an ELISA plate reader (BioTek Instruments, Inc.). The intensity of the color developed was inversely proportional to the concentration of the hormones in the plasma. A standard curve was plotted using the standards provided with the kit. The concentration of each hormone in the samples was interpolated from the standard curve. The intra- and inter-coefficients of variation were <6 and 8%, respectively. Each plasma test sample and standard was run in duplicate.
Six birds from each group were sacrificed at 28 and 34 weeks of age by cervical dislocation for collection of the jejunum (from the pancreatic loop to Meckel’s diverticulum) and magnum tissues (portion of the oviduct). After collection, the samples were transported to the laboratory in saline. The tissues were rinsed in PBS and placed on moistened paper towels, and any adhering fat and connective tissue were removed. The samples were immediately stored at -80 °C for further analysis. Gene expression studies were conducted on both the jejunum and magnum tissues of the birds.
The mean ± SEM values for essential amino acids were calculated similarly to the values for hormones. The total free plasma amino acids were analyzed on HPLC equipment (WATERS Alliance Separations module 2695). Only 19 amino acids could be analyzed. One hundred microliters of each plasma sample was taken, and 400 µl of methanol was added to precipitate the proteins. The samples were incubated overnight at -20 °C. After centrifugation at 4000 rpm for 30 min, the supernatant was transferred to another tube. The supernatant was evaporated under N2 at 60 °C to dry it completely. Derivatizing reagent was added to the sample, which was then incubated for 60 min at 45 °C and vacuum evaporated. The resultant pellet was dissolved in 100 µl of Buffer-A. The mixture was vortexed and centrifuged at 13000 rpm for 15 min, and the supernatant was collected in vials. A total of 20 µl was loaded onto the instrument, which was quantified using standards purchased from Sigma Co., USA. The column used was a Luna C18 column (250 × 4.6 mm; 5 µl). The flow rate was 1 ml/min. The gradient run time was 80 min. The mobile stage A used was sodium acetate buffer, and the mobile stage B used was buffer A + acetonitrile. The test samples were run in triplicate.
Peak area of AA in Test x Concentration of AA Standard (pm)
Peak area of AA in the standard
X
Molecular Weight of AA
Concentration of Test Sample (µl) × 103
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA), purified with an RNeasy Mini Kit (Qiagen, Valencia, CA), and treated with an RNAse-Free DNAse kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The RNA was suspended in diethyl pyrocarbonated (DEPC)-treated water, and the sample purity and concentration were measured on a NanoDrop 2000 Spectrophotometer (Thermo Scientific, Wilmington, DE) and stored at -80 °C. The purity of the extracted RNA was assessed by UV absorbance, and the OD260/280 ratios for all the samples were ≥1.9. The RNA concentrations ranged from 1.0 to 1.5 μg/μl. Total RNA (2 μg) was reverse transcribed with a cDNA reverse transcription kit according to the manufacturer’s protocol (Thermo Scientific, Verso cDNA synthesis kit) using a Gradient Master Cycler (Eppendorf, Hauppauge, NY). The cDNA samples were stored at -20 °C. cDNA samples were diluted 1:1 prior to RT‒PCR analysis. Each reaction consisted of 1 μL of diluted cDNA, 1.0 μL of forward primer, 1.0 μL of reverse primer, 7 μL of DEPC water, and 10 μL of Maxima SYBR Green/ROX qPCR Master Mix (2×) (Thermo Fisher
Scientific). The genes and sequences of primers used for the RT‒ PCR assays are listed in Table 2. The primers were synthesized by Chromous Co. Bangalore, India. The qPCR conditions were 95 °C for 5 min, followed by 40 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s. Both the control and treatment values were normalized against the b-actin reference gene, and further treatment values were normalized against the control. The relative quantification (RQ) was expressed as the ratio of the target gene to the reference gene (b-Actin) using the delta-delta Ct (∆∆ Ct) method (23). All samples were run in triplicate.
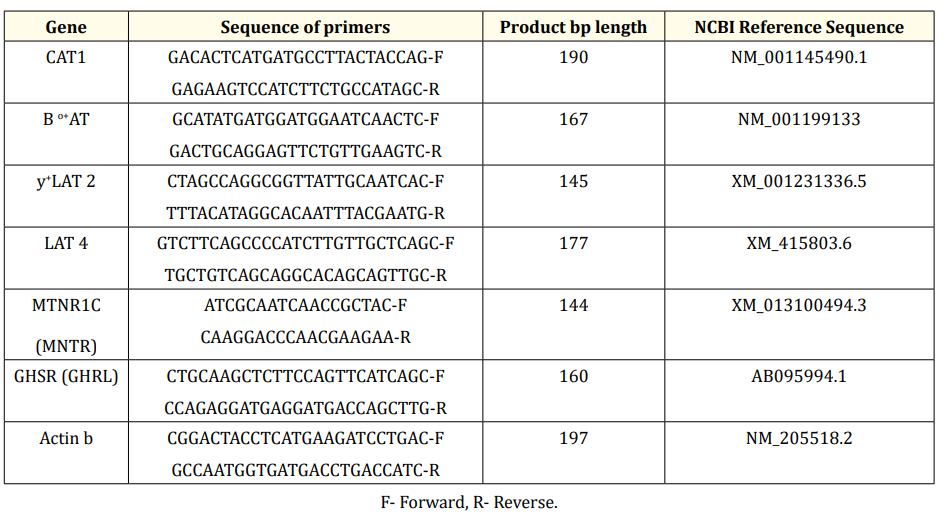
Table 2: Primer sequences of the genes.
F- Forward, R- Reverse.
The total Se content of the basal diet (Fresh) and treatment diet was analyzed using inductively coupled plasma‒mass spectrometry (ICP-MS; Agilent 7500cx, Agilent Technologies, Tokyo, Japan).
Body weight was recorded at fortnight intervals during the course of the study. The mean ± SE values were estimated for twenty birds from each group.
The rate of egg production for a week was calculated as the total number of eggs for a week/actual no. of hens × 100 days.
Actual no. of hen days = Total number of hens in a week - loss of hens in that week.
For estimation of egg weight, 20 eggs were collected randomly every day from each group, the eggs were weighed on an electronic balance to the nearest g, and the mean values were subsequently calculated.
All data were statistically analyzed using the SPSS statistical software for Windows (Version12.0; SPSS Chicago, Illinois). The experimental data are presented as mean ± SEM. The means of independent samples of two groups were compared by unpaired two sample t test for different hormones, amino acids and gene expression parameters at different stages of laying period. The data was screened for outliers and it was ensured that the values are normally distributed. In order to check for homogeneity of variance, Levene’s test for equality of variances was performed. Thereafter, the significant differences (if any) between the means of control and treatment groups for the parameters were identified using unpaired two sample t-test. The t value was calculated using the t test formula for independent samples with homogenous variance as follows:
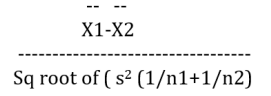
Where X1 = Mean of first group
X2 = Mean of second group
n1 = Sample size of first group
n2 = Sample size of second group
s = standard deviation of the groups
The means were considered to be statistically different at P < 0.05.
The results for each parameter are given for the two stages, namely, the early (EP stage, 26-30 weeks) and middle stages (MP stage, 32-36 weeks), of the laying period. The mean ± SEM values of MET and GHL were compared between the control groups (CGs, basal feed 0 SY, Table 1) in the EP and MP stages, and it was observed that the circulatory levels of GHL were significantly (P < 0.01) greater in the EP stage than in the MP stage (Table 3). When comparing the two stages, the differences in the concentrations of MET and PROG in the CGs were not significant. However, the mean values of EST (P < 0.001) were greater at the MP site than at the EP site (Table 3). Similarly, the mean ± SEM values of all hormones for each stage were compared between the control (CG, 0) and treatment groups (TG, 0.2); the mean ± SEM values for the MET hormone at the EP were not significantly different (P>0.05), whereas the TG concentration was greater during the MP treatment (P < 0.01) (Table 3). The same treatment significantly (P < 0.001) decreased the concentration of GHL at the EP and increased it at the MP (Table 3). The plasma EST concentration in the TG increased significantly (P < 0.001) at both the EP and MP stages (Table 3). When compared to the respective values in the CG, the concentration of PROG decreased (P < 0.01) at the EP stage and increased (P < 0.01) at the MP stage in the TG (Table 3).
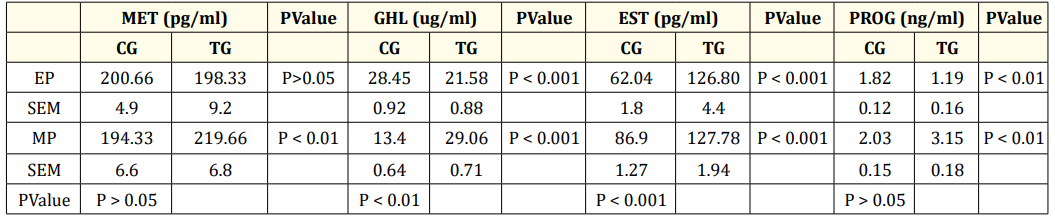
Table 3: Concentration of Plasma hormones during early and mid-phase of laying period.
Values are represented as Mean ± SEM, MET-Melatonin, GHL-Ghrelin, EST-Estrogen, PROG-Progesterone; EP-Early phase (26-30 weeks); MP-mid phase (32-36 weeks). CG- control group, (0 ppm of organic SY), TG-Treatment group (0.2ppm of SY). Significance between control groups of EP and MP is given column wise. Significant values between control and treatment groups with in EP and MP is given row wise. N = 6.
At the EP stage, the fold change in the expression of the ghrelin receptor (GHRL) in the TG decreased significantly (P < 0.01) in both the jejunum (Figure 1a) and magnum (Figure 1b), with a decrease in the concentration of plasma GHL (Table 3) compared to that in the CG jejunum and magnum. Treatment with SY decreased (P < 0.01) the expression of the melatonin receptor (MNTR) in the magnum (Figure 1b), indicating that circulatory levels at the EP stage may require less MET activity in magnum tissue. The expression of the MNTR was greater in the jejunum (P < 0.001) and lower in the magnum (P < 0.01) in the TG than in the CG. At the MP stage, both MNTR and GHRL were expressed at lower levels (P < 0.01) in the jejunum tissue of the TG than in that of the CG (Figure 2a). However, the fold change in the expression of only MNTR increased significantly (P < 0.01), without any significant change in the expression of GHLR in the magnum of the TG compared to in that of the CG (Figure 2b). Comparing the EP and the MP stages of the TG revealed that the treatment increased the fold change in the expression of MNTR (P < 0.01) and GHRL in the magnum at the MP stage (Figure 1b and 2b). This coincided with the increase in the plasma levels of hormones, resulting in increased egg production at a later stage.
When comparing the EP and the MP stages of the CG, the number of plasma essential amino acids whose concentration was higher or lower was more or less the same. The mean concentrations of six nonessential amino acids (Table 4a) and 4/8 essential amino acids (Table 4a) were significantly greater during the MP than during the EP stage. The concentrations of the two nonessential compounds were not significantly different from each other between the CG EP and MP stages. Hence, the levels of the remaining essential amino acids (4) (Table 4a) and three nonessential amino acids significantly decreased at the MP stage (Table 4a). Comparing the CG and TG at the EP stage revealed that in the TG, the concentrations of VAL, TRP (P < 0.001) and ARG, LYS decreased (P < 0.01), whereas the concentrations of LEU, THRE, and MET did not change significantly (P>0.05) (Table 4b). The concentration of IsoLeu (P < 0.001) increased only at the EP stage. However, during the MP treatment, SY significantly decreased the plasma concentrations of MET, ARG, THRE, IsoLeu, VAL, TRP, and LYS at different levels in the MP stage treatment (Table 4c) compared to the concentrations in the CG treatment. Compared with the plasma concentrations in the CG, the concentrations of the plasma nonessential amino acids ASG, GLUT acid and HIS in the TG increased significantly (P < 0.001), whereas the concentrations of the remaining eight plasma amino acids decreased significantly (Table 4c). The concentrations of more essential amino acids decreased at both the EP (4) and MP (7) stages (Table 4b&c), and the concentrations of nonessential amino acids decreased at the EP (7) and MP (8) stages (Table 4b&c) in the TG, suggesting greater utilization of amino acids at the MP stage. As the birds passed from the early to mid-stages, the treatment did not significantly affect the increase in body or egg weight (Table 5), but did affect egg production (Table 5).
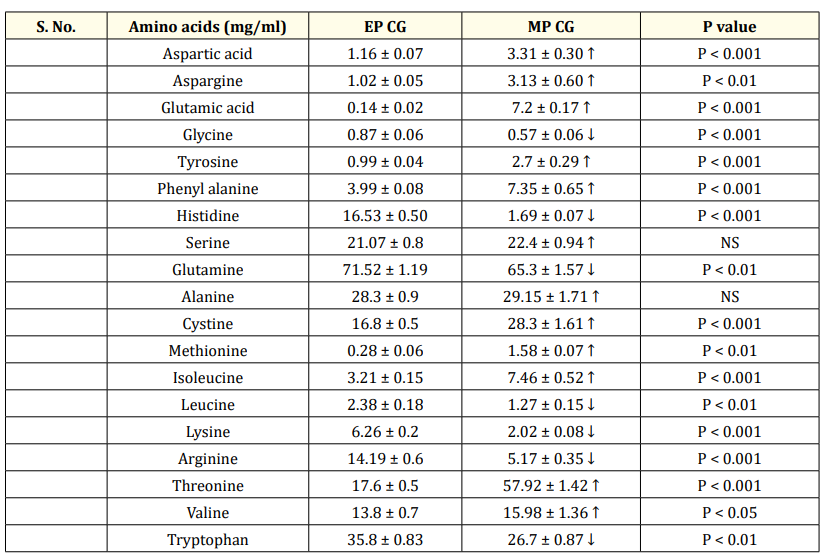
Table 4: (a) Comparison of concentration of plasma amino acids between CGs of EP and MP.
Values are represesented as Mean ± SEM. CG- control groups. EP- Early phase (26-30 weeks); MP-mid phase (32-36 weeks.) N = 6. CG- control group, (0 ppm of organic SY), Essential amino acids are represented as fonts in bold. ↑ Increase, ↓ decrease.
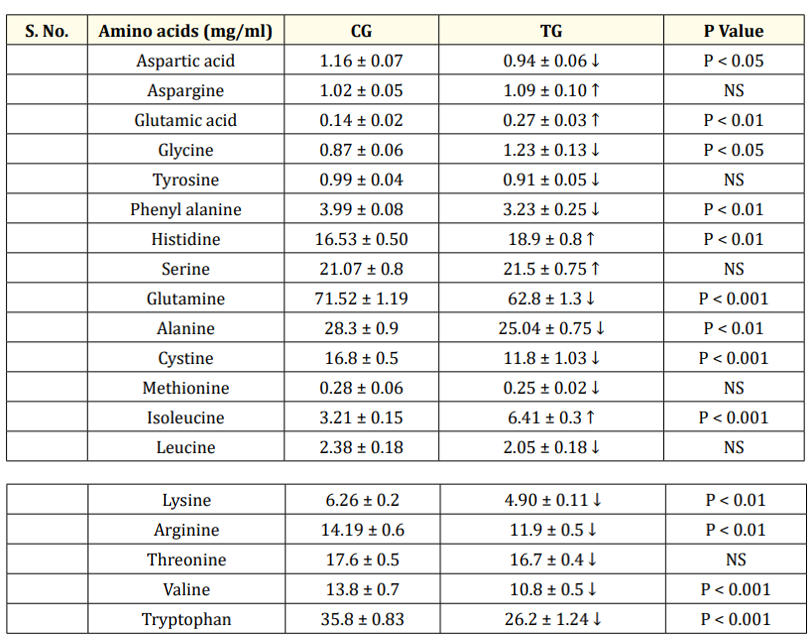
Table 4b: Comparison of concentration of plasma amino acids between CG and TG at EP.
Values are represented as Mean ± SEM. a) EP-Early phase (26-28 weeks). CG- control group, (0 ppm of organic SY), TG-Treatment group (0.2ppm of SY), NS- Not Significant, N = 6. Essential amino acids represented as bold faced fonts. ↑ Increase, ↓ decrease.
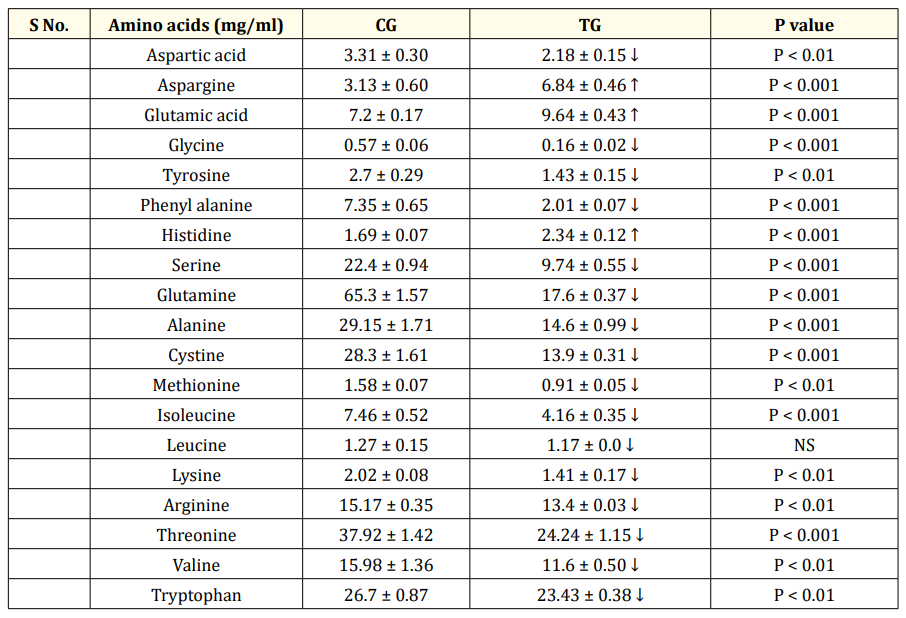
Table 4c: Comparison of concentration of plasma amino acids between CG and TG at MP.
Values are represented as Mean ± SEM. MP-mid phase (32-36 weeks). CG- control group, (0 ppm of organic SY), TG-Treatment group (0.2ppm of SY), Significant values between control and treatment groups MP NS- Not Significant, N = 6. Essential amino acids represented as bold faced fonts. ↑ Increase, ↓ decrease.
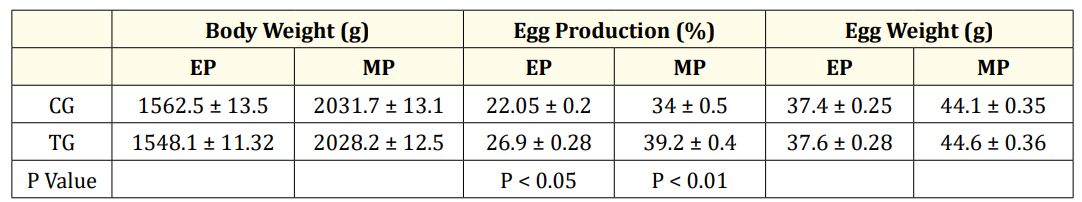
Table 5: Body weight, Egg production and weights at Early and Mid-laying period.
Values are represented as Mean ± SEM. EP-Early phase (26-30 weeks); MP-mid phase (32-36 weeks).CG- control group, (0 ppm of organic SY), TG-Treatment group 0.2ppm of SY. Significant values between control and treatment groups with in EP and MP is given column wise. N = 15.
At the EP stage, the expression of the jejunum tissue amino acid transporters (aats) LAT4 (P < 0.05) and BAT decreased (P < 0.01) in the TG (Figure 1a) compared to that in the CG. However, in the magnum of the TG, the fold changes in the expression of the transporters CAT (P < 0.01), BAT (P < 0.001), and LAT2 (P < 0.001) increased significantly (Figure 1b) compared to those in the CG. The concentration of total plasma amino acids (Table 4a.) in the CG was greater at the MP stage than at the EP stage (Table 4a). Treatment at the MP stage, only LAT2 expression (P < 0.001) significantly increased and decreased (P < 0.01) the expression of CAT and BAT in the jejunum (Figure 2a). In the magnum tissue, the expression of LAT4 only increased (P < 0.001), with no significant change in the expression of other aats (Figure 2b).
Since the concentrations of plasma HIS and isoleucine increased significantly (Table 4b), the expression of the neutral amino acid transporters BAT and LAT4 might have decreased in the jejunum at the EP stage, where the absorption of amino acids occurs. The concentration of most plasma amino acids with respect to total plasma amino acids was lower in the TG group than in the CG group during the EP and MP stages (Table 4b&c), possibly due to increased transport to other tissues. However, the body weight in the TG did not change significantly at either the EP or MP stage (Table 5).
The concentration of selenium in the CG was 0.28 ± 0.05 ppm, and that in the TG was 0.46 ± 0.04 ppm.
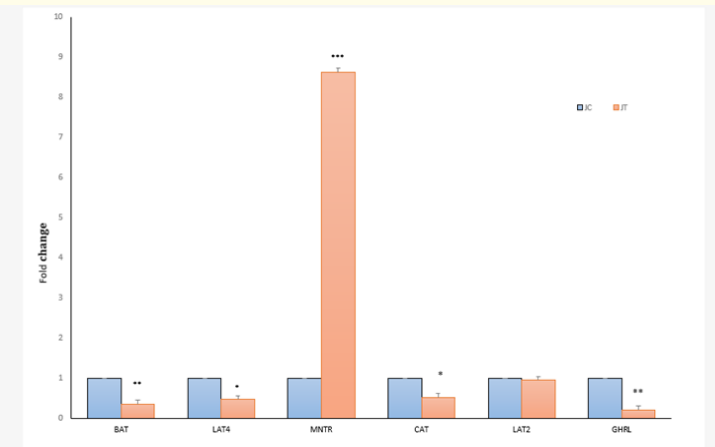
Figure 1a: Fold change expression of aminio acid transporters and hormone receptors MNTR and GHRL in jejunum (J) tissues at 26 weeks (EP) between Control (C,0) and Treatment (T,0.2ppm) groups. *P < 0.05, **P < 0.01, *** P < 0.001.Value of C = 1. N = 6.
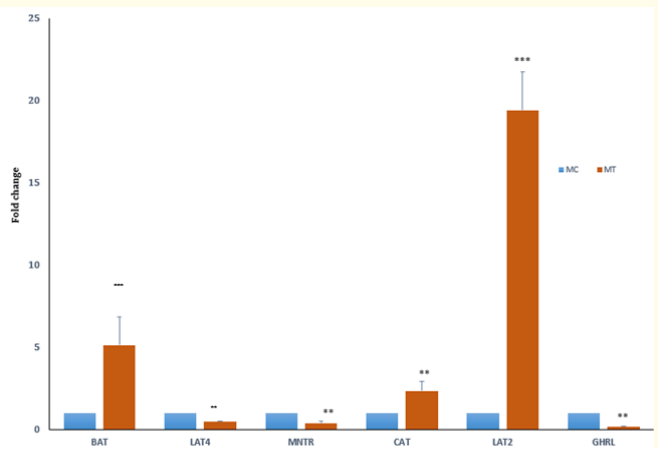
Figure 1b: Fold change expression of amino acid transporters and hormone receptors in magnum tissues at EP (26 weeks) between control (C, 0 SY) and supplemented (T, 0.2ppm SY) groups of Aseel chickens. ** P < 0.01,***P < 0.001.Control = 1, N = 6.
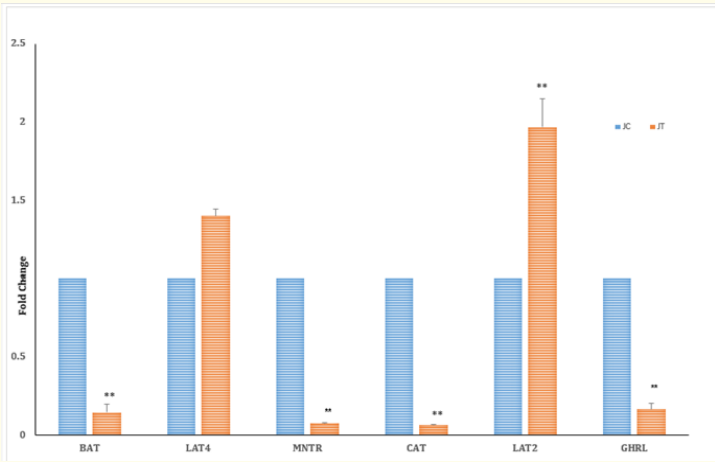
Figure 2a: Fold change expression of aminio acid transporters and hormone receptors MNTR and GHRL in jejunum tissues at 32 weeks (MP) between Control (C,0) and Treatment (T,0.2ppm) groups . **P < 0.01. C = 1, N = 6.
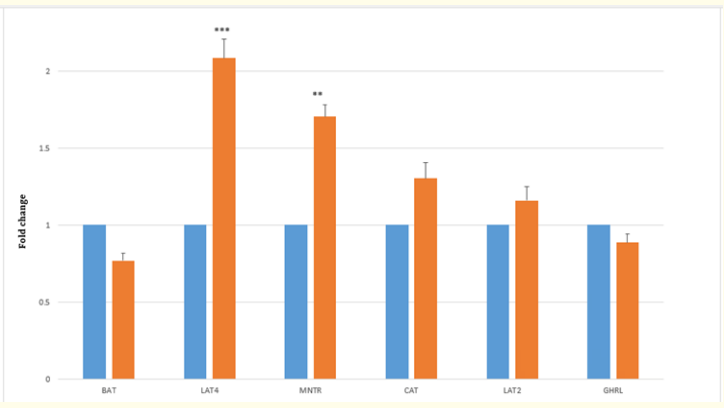
Figure 2b: Fold change in the expression of amino acid transporters and hormone receptors (MNTR) and (GHRL) between Control (C,0) and Treatment (T,0.2ppm) groups in magnum tissues at MP (34 weeks). **P < 0.01, ***P < 0.001. Value of C = 1, N = 6.
In the present study, at the EP stage, treatment with SY decreased plasma melatonin, but not significantly. However, Li., et al. (2015) reported that a decrease in plasma MET is also favorable for egg production. Moreover, MET enhances the laying rate and follicle growth [25]. Hence, in the present experiment, during the EP stage, when Aseel hens started laying eggs, the availability of plasma MET may have been greater in the CG; hence, treatment did not have any effect on the concentration of MET but increased egg production. Circulatory levels of GHL also depend on the levels of circulating EST hormone [26]. In the present study, a comparison of the CG EP and MP stages revealed that the GHL concentration was greater and the EST hormone concentration was lower at the EP stage. An inverse relationship was observed between the GHL and EST levels at the EP stage only, whereas at the MP stage, the treatment effect on both hormones was positive. Circulatory levels of ghrelin are dependent on the level of steroid hormones [25,27]. Se can directly affect the proliferation of ovarian cells and the production of EST [28]. Hence, the direct effect of SY resulting in increased concentrations of EST at both the EP and MP stages in the TG cannot be ruled out. Selenium treatment has been shown to have stimulatory effects on the circulatory levels of EST. It has also been reported that when in vitro studies were conducted with luteinized granulosa cells, treatment with selenium increased estradiol secretion from granulosa cell cultures [29]. The inhibitory effect of GHL on progesterone secretion from porcine and human luteal cells in vitro has also been reported [13,30]. A similar inverse relationship was observed between GHL and PROG in both the EP and MP stage of the CG. At the EP stage, the hormone levels indicated that sufficient amounts of circulatory hormones are present, and a decrease in plasma levels as well as at the receptor level might increase egg production. Circulatory levels of GHL also depend on the levels of circulating EST hormone [26]. With 0.2 mg of Se, the dose may be greater for chickens at the EP stage when egg production is lower and the utilization of hormones is also lower. This dose may be more effective for the positive modulation of physiological parameters at the EP stage when sufficient concentrations of hormones other than EST are available. The results in this study also indicated that selenium is able to maintain homeostasis with respect to hormone levels. The jejunum is more sensitive to MET at the EP stage. Treatment with Se revealed that jejunum tissue is less sensitive than normal tissue to increased levels of circulatory GHL or MET at the MP stage. The results with respect to the MNTR indicated that the jejunum became more sensitive to MET at the EP stage and magnum during the MP stage.
The relationship between circulatory MET and GHL and amino acids in the TG revealed that jejunum tissue is less sensitive to increased levels of circulatory GHL or MET at the MP stage. The decrease in the concentration of more essential amino acids at both the EP (4) and MP (7) stages and in the concentration of nonessential amino acids at both the EP (7) and MP (8) stages suggested greater utilization of amino acids in the TG at the MP stage. This coincided with the greater increase in the rate of egg production after treatment with SY during the MP stage than during the EP stage. Treatment might have improved the utilization of amino acids for production. The trace mineral selenium can affect the growth and proliferation of follicles [31]. Since the concentration of total plasma amino acids in the CG was greater at the MP stage [12,19], treatment might have decreased the concentration and increased the utilization, resulting in increased egg production.
At the EP stage, the decrease in the expression of aats in the jejunum in the TG indicated sufficient levels of aas in the jejunum. In the magnum, the increase in the number of aats indicated greater transport of plasma aas to the magnum. Since the concentrations of more amino acids decreased at the EP or MP stage in the TG treatment compared to the CG, the expression of aats was greater in the magnum at both stages. Since the concentration of total plasma aas was greater at the MP stage than at the EP stage of the CG, treatment with Se might not have increased the number of aats in the magnum. This finding also suggested that treatment with selenium might have caused a sufficient concentration of aas in the CG magnum tissue at the MP stage. In the magnum, the expression of amino acid transporters increased in response to supplementation, and there was a greater decrease in the level of amino acids which was directly related to an increase in the rate of egg production during the EP stage.
In the present study, the transport of cationic amino acids might have been reduced due to a reduction in the expression of CAT and BAT. The L-amino acid transporter system is a major nutrient transport system that is responsible for Na+-independent transport of neutral amino acids, including several essential amino acids. Although we have not conducted studies on the presence of Na ions, the L system might have compensated for the reduction in the expression of the y+ transport system (BAT and CAT). Homeostasis is maintained in physiological systems, whether via metabolites or amino acids. Transporters primarily determine amino acid equilibrium concentrations because their uptake and efflux rates are much faster than those of metabolism and protein synthesis [32]. This indicates that at the MP stage, the concentration of amino acids in the tissues may increase; hence, significantly fewer aats were detected in the tissues. Selenium is able to maintain homeostasis and can influence egg production positively.
This study indicated that the availability of amino acids in the jejunum was sufficient at the EP stage, and subsequently, the availability of amino acids in the magnum might have also increased at the MP stage. Both CG and TG homeostasis are maintained between circulatory amino acids and aats. This in turn significantly increased egg production in both stages. The SY may also have a direct effect on the functions of magnum tissue and other parts of the reproductive tract, leading to increased egg production. An increase in egg production in response to treatment with selenium in hens has also been reported by Lv., et al. (2019). The egg production of the CG increased from 22-34% as the birds passed from the EP to the MP stage. Treatment with Se significantly increased the fold change in the expression of only the large amino acid transporters LAT4 and LAT2 in the jejunum and LAT4 in the magnum compared to that in the CG tissues at the MP stage. The expression of only some aats was sufficient for increasing egg production (5%, TG) at the MP stage. Treatment with SY suitably modulated the level of hormones at the EP stage as well as at the MP stage, and greater utilization of amino acids increased egg production.
From the present study, it can be concluded that the physiological circulatory levels of hormones differ between the early and mid-stages, as does the concentration of plasma amino acids. Treatment with an additional 0.2 ppm organic selenium at the EP or MP stage can modulate the above mentioned endocrine and associated molecular parameters, which in turn can increase the rate of egg production in the native Aseel breed.
Acknowledgements are due to Indian Council of Agricultural Research, New Delhi, India for providing financial support for conducting the studies.
The authors declare no conflict of interest.
Copyright: © 2024 Anand Laxmi Nidamanuri., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.