Danais Vidal Rossell1* and Jani Laffitte García2
1Level I Technologist, Molecular Immunology Center, Havana, Cuba
2Molecular Immunology Center, Havana, Cuba
*Corresponding Author: Danais Vidal Rossell, Level I Technologist, Molecular Immunology Center, Havana, Cuba.
Received: October 03, 2024; Published: October 18, 2024
Citation: Danais Vidal Rossell and Jani Laffitte García. “Physicochemical Methods I-number and Ibio-number Based on Capillary Electrophoresis as Predictors of Erythropoietin Bioactivity". Acta Scientific Pharmaceutical Sciences 8.11 (2024):03-09.
Mathematical models I-number and Ibio-number based on capillary zone electrophoresis profile have been used to compare the bioactivity of erythropoietin in international standards. The replacement of the biological activity method in mice described in the European Pharmacopoeia for the Active Pharmaceutical Ingredient of the product, by a physicochemical method, has great advantages, ranging from the solution of the ethical problem of consuming thousands of experimental mice for batch release, to an increase in accuracy and potency measurement, which guarantees its safety and efficacy. The aim of the work was to develop the I-number and Ibio-number methods in two production facilities of the Molecular Immunology Center to determine the bioactivity of erythropoietin. The method was set up by analyzing the International Physicochemical Standard of Erythropoietin (CRS 1.0). The biological activity of 10 batches of Active Pharmaceutical Ingredient produced in each facility was determined by in vivo testing using the I-number and Ibio-number models. A variability of ˂ 1% and an accuracy of 99% of the estimated potency were obtained using both models for CRS 1.0. An accuracy of the bioactivity of the batches produced determined by both models was found to be between 107% and 120%, taking as the “true” value the potency calculated in the in vivo test. The I-number and Ibio-number methods were developed and shown to be suitable for replacing the potency assay in mice.
Keywords: I-number; Ibio-number; Erythropoietin; Capillary Electrophoresis; Bioactivity
At the Center for Molecular Immunology (CIM), recombinant human erythropoietin (rhEPO) type alpha has been produced for more than 25 years from the cloning of the human gene for the protein in Chinese hamster ovary cells (CHO) under good production practice standards. rhEPO is a glycoprotein hormone that stimulates the proliferation of erythroid cells, which is why it has wide utility as a therapy in diseases such as chronic renal failure, aplastic anemia, in diseases associated with low levels of erythropoietin in plasma and as a complement in therapies for high incidence diseases, such as cancer and AIDS [1].
In the quality control process of this drug, both for the production process, as well as for the Active Pharmaceutical Ingredient (API) and the finished product; the quality attributes of the protein that ensure its safety and efficacy are checked. As critical quality attributes, the identity, purity, strength and biological activity of the protein are controlled together with the determination of the presence of contaminants in the process, using a battery of analytical techniques that are described in international drug monographs such as the European Pharmacopoeia (Ph.Eur.) [2]. The biological activity of rhEPO, according to the Ph. Eur., is evaluated by an in vivo test based on the measurement of reticulocyte stimulation in normocythemic mice [2], which by its nature, is considered very expensive due to the use of a large number of experimental animals, highly variable (CV ~ 30%) and inaccurate (~70% -130%) [3].
The 3Rs Principle (Refinement, Reduction and Replacement) of bioassays by more accurate and precise methods such as physicochemical methods, cell-based methods or other alternatives; is currently a common goal of European communities, animal welfare societies, expert groups, regulatory authorities and pharmaceutical companies; in particular for RhEPO [4]. Due to this, in recent years the scientific community has focused on finding alternatives for the replacement of the mouse assay to determine the biological activity of RhEPO.
At present, the structure-function relationship of RhEPO is well established. Its mechanism of action is based on the binding of RhEPO to its receptor, which triggers an intracellular signaling pathway highly dependent on the amino acid sequence of the protein and its post-translational modifications (glycosylation and glycan modifications) [5]. It has been shown that glycosylation has a high impact on the biological activity of proteins and is fundamentally associated with their stability, solubility, folding and half-life [6]. The glycosylation pattern of the protein isoforms determines the profile obtained by identification using the capillary zone electrophoresis (CZE) method, so this in turn has a direct relationship with the bioactivity of the protein [7]. The capillary zone electrophoresis profile of rhEPO is characterized by the presence of up to 8 different isoforms, whose area percentages are specified in the Ph. Eur. for each of the isoforms [2].
On the other hand, the bioactivities of the individual isoforms have been published by the company Amgen ® in 1996 for the alpha type RhEPO [8] and for the beta type RhEPO by the company Roche® in 2014; [9] which has led to the description of mathematical models to establish the structure-function relationship of this protein based on its isoform profile and the bioactivity of the molecule. In recent years, based on this approach, the Z-number assay based on ion exchange chromatography for glycan mapping has been published [10], and Capillary zone electrophoresis-based assays: I-number [11], Ibio-number [12] and the reduced linear regression model. The latter has been used to determine the bioactivity of rhEPO beta produced by Roche® and there is evidence of its approval by the European Medicines Agency for quality control analysis of the product in Europe [13]. Although it has been shown that the use of I-number assays and Ibio-number allows to accurately calculate the bioactivity of different erythropoietin reference standards [14], there are no reports of its use in the quality control of API and in particular of rhEPO alpha. The objective of the present work was to develop the I-number and Ibio-number methods in two production facilities of the Molecular Immunology Center to determine the bioactivity of the erythropoietin API.
The study was carried out in two CIM production facilities located in two different countries. Sample analysis was performed in the quality control laboratories of each production facility. In both cases, the ECZ method described in Ph. Eur. was used for protein identification [2] and a PA 800 plus capillary electrophoresis equipment (Beckman Coulter, USA) and the 32 Karat ® version 10.1 equipment operation and data analysis program were used. For the assembly and development of the I-number and Ibio-number methods, lot 1.0 of the International Physical-Chemical Reference Standard of Ph. Eur. (CRS, for its acronym in English, Chemical Reference Standard) was used, which contains a 1:1 mixture of epoetin alpha:beta. CRS 1.0 was established as the Ph. Eur. erythropoietin standard. in 2015 and was prepared from batch 3 of the Biological Reference Preparation (BRP) [15].
For the analysis of rhEPO API batches, 10 batches produced in each of the CIM production facilities and previously released by the Quality Assurance Department were used. In both cases, the API contains rhEPO type alpha ≥ 0.5 mg/mL, 5.8 mg/mL sodium citrate, 5.8 mg/mL sodium chloride, 0.069 mg/mL citric acid and 0.220 mg/mL polysorbate 20 dissolved in water for injection.
The determination of the in vivo biological activity of the IFA batches was carried out using the in vivo method in normocythemic mice described in the Ph. Eur [2].
The I-number (I) was defined as the sum of the products of the area percentage of each isoform (pn) and the corresponding isoform number (n) taking into account the eight isoforms of the RhEPO profile as shown in equation 1.
I = p1 × 1 + p2 × 2 + p3 × 3 + p4 × 4+ p5 × 5+ p6 × 6 + p7 × 7+ p8 × 8 (1)
The bioactivity by the I-number method for CRS 1.0 was calculated using equation 2, from the I-number value determined for CRS 1.0 in the present study, the certified bioactivity for lot 3 of the international biological standard of erythropoietin (BRP, Biological Reference Preparation): (141.1 IU/µ g) [16] and the I-number value reported by Hermentin in 2017 for BRP 3: (530.9) [11].
Bioactivity (CRS 1.0) = I (CRS 1.0) /I (BRP 3) * Bioactivity (BRP 3) (2)
For the determination of the bioactivity of the IFA batches produced, equation 3 was used, based on the I-number value calculated for the CRS 1.0 in this study, the certified bioactivity for it (141.5 IU/mg) [16] and the I-number value determined for each IFA batch in the present study.
Bioactividad (IFA) = I (IFA)/I (CRS 1.0) * Bioactividad (CRS 1.0) (3)
For the development of the I-number method, the obtaining of the CRS 1.0 type profile was verified in five different runs and compliance with the specifications according to Ph. Eur. This method has as its starting point the determination of the percentage of individual isoforms from the ECZ profile which, being a compendial method, according to the current regulations for validation of analytical methods ICH Q2(R2), does not require a total validation; but a verification of the same must be carried out using an international reference standard under the analytical conditions of each laboratory [17].
The accuracy of the determination of the I-number value under intermediate precision conditions and the accuracy of the bioactivity estimated by both methods were studied, based on the certified biological activity value for the international standard CRS 1.0.
The Ibio-number (Ibio) was defined as the sum of the products of the percentage parts of the individual peak area (pn) obtained by ECZ and the isoform factors (F i) corresponding to the bioactivities of the respective isoforms (equation 4).
Ibio = p1 × F1 + p2 × F2 + p3 × F3 + p4 × F4 + p5 × F5 + p6 × F6 + p7 × F7 + p8 × F8 (4)
For epoetin alfa and epoetin beta, the Fi factors were derived from the bioactivities of the individual isoforms as published by Amgen ® for epoetin alfa [8] which allowed the calculation of the value of Ibio alpha, and by Roche ® for epoetin beta [9] to calculate the Ibio beta value. The bioactivities of the isoforms were published as "polypeptide units/mg of erythropoietin" for RhEPO alpha [8] and as "IU/mg of protein" for RhEPO beta [9], therefore in this study the Ibio alpha and Ibio beta values were multiplied by the correction factor 0.60 (polypeptide/mg of erythropoietin glycoprotein) to express the bioactivities in potency units/mg of erythropoietin glycoprotein. The "final" Ibio values were calculated as an arithmetic mean from the Ibio alpha and Ibio beta values. beta. Isoforms 1-8 (where applicable) were used for the calculation of the Ibio number.
For the development of the Ibio-number method, the obtaining of the CRS 1.0 type profile was verified in five different runs and compliance with the specifications according to Ph. Eur. was analyzed.
Ibio-number value under intermediate precision conditions and the accuracy of the bioactivity estimated by both methods were studied, based on the certified biological activity value for the international standard CRS 1.0.
The biological activity of 10 batches of RhEPO alpha API produced in each of the production facilities was evaluated using the I-number, Ibio-number assays and the in vivo assay in normocythemic mice. The accuracy of the bioactivity determined from the I-number and Ibio-number assays was verified by taking the biological activity values of the batches determined by the in vivo assay as the “true” value and the difference found between the different methods was analyzed.
A typical profile for CRS 1.0 was obtained in the five runs performed using the CZE method in the quality control laboratories of both production facilities (Figure 1), similar to each other and to the electropherogram described in the quality certificate for CRS 1.0. [15] In all cases, the runs complied with the system suitability tests established for this method in the Ph. Eur [2].

Figure 1: Standard electropherogram of the International Physicochemical Reference Standard for Erythropoietin (CRS 1.0) obtained in both production facilities by capillary zone electrophoresis (obtained by the author).
The quantitative analysis performed for the area percentage of the separated individual isoforms shows that in all cases the ranges specified for isoforms 1-8 were met, as shown in Table 1.
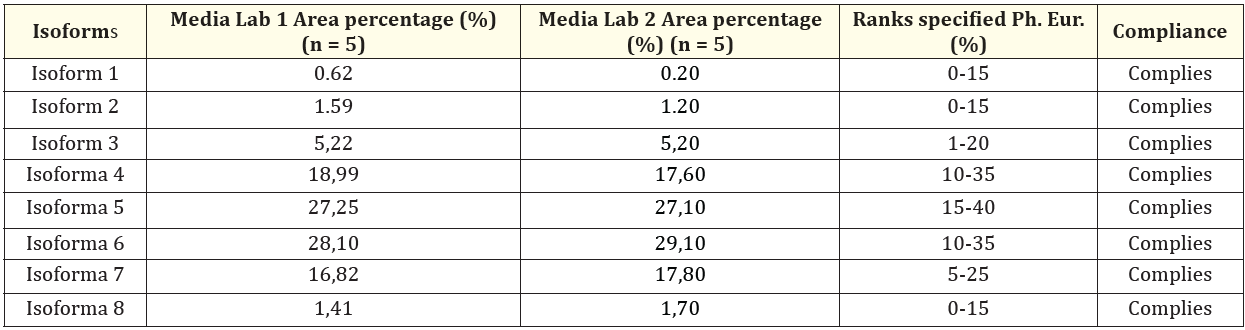
Table 1: Isoform percentages obtained for the Physical-Chemical Reference Standard international erythropoietin level (CRS 1.0) by electrophoresis capillary zone in both production facilities.
The results are shown in Table 2 obtained in both facilities productive for the CRS 1.0 by I -number method.

Table 2: Results of the I-number method for the Physicochemical Reference Standard international erythropoietin (CRS 1.0) at both facilities productive.
They were obtained I -number values very similar for CRS 1.0 in both facilities productive. Likewise, were determined bioactivity values similar with similar variability and accuracy of measurement in both cases.
The results are shown in Table 3 obtained in both facilities productive for the CRS 1.0 by Ibio-number method.

Table 3: Results of the Ibio-number method for the Physicochemical Reference Standard international erythropoietin (CRS 1.0) at both facilities productive.
Bioactivity determined through this method was very similar for CRS 1.0 in both facilities productive and values were determined with similar variability and accuracy in both cases.
Evaluation of biological activity in batches of RhEPO produced in both production facilities.
Table 4 shows the results obtained in both production facilities for the evaluation of the RhEPO alpha batches produced using the I-number, I-bio number methods and in normocythemic mice.
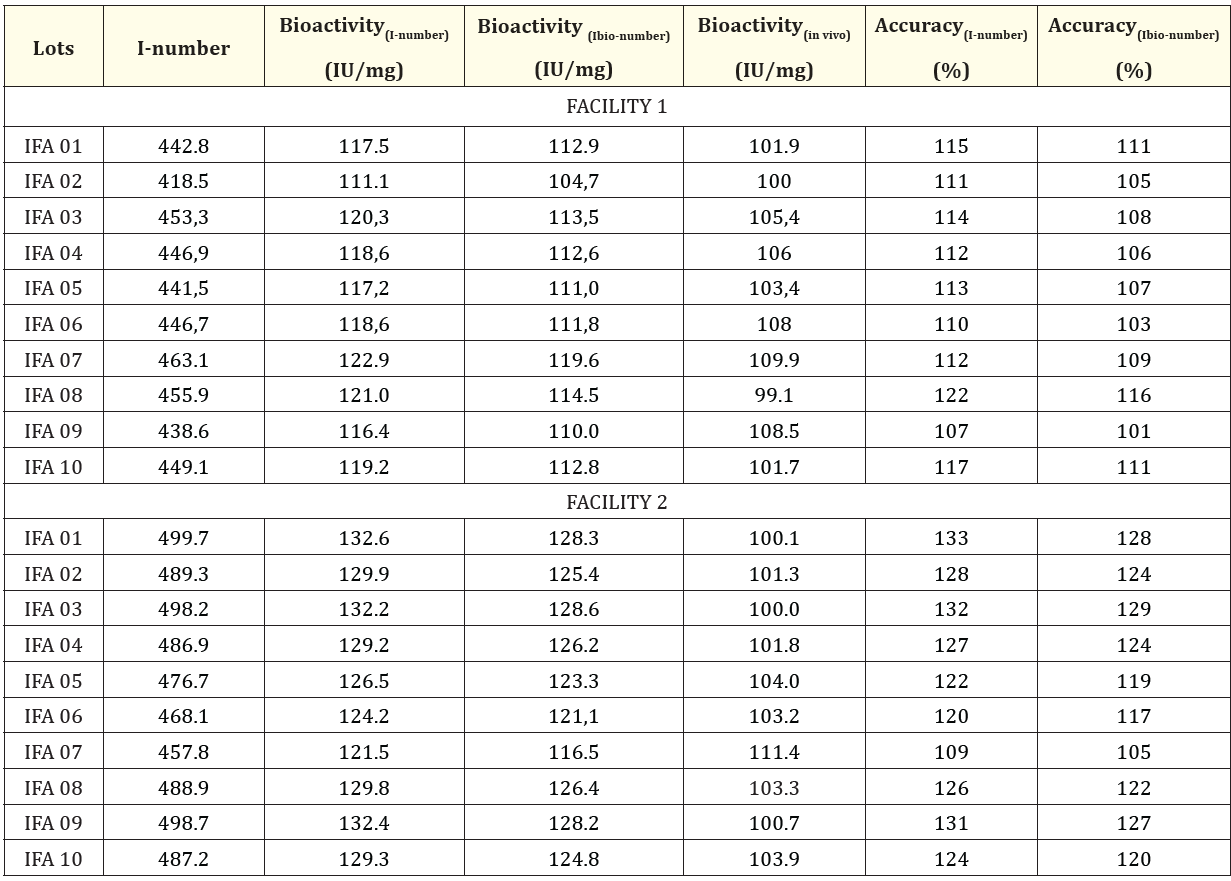
Table 4: Bioactivity evaluated in batches of RhEPO alpha produced in both production facilities and measurement accuracy
In all cases, the potency specification established in the Ph. Eur. for the erythropoietin API was met (not less than 100,000 IU/mg of protein) [2].
On the other hand, the accuracy of the bioactivity determined by the models based on the ECZ profile (Table 4) was determined, assuming as “true value” the bioactivity determined by the in vivo test; since despite its limitations in terms of precision and accuracy of the measurement being reported [19], it is the established method in the Ph. Eur. for the evaluation of the potency in the IFA of the product. An accuracy was found in facility 1 between 107% and 117% for the I-number method. and between 101% and 116% for the Ibio-number method and in facility 2 it was between 109% and 132% for the I-number method and between 105% and 129% for the Ibio-number method.
Obtaining the standard electropherograms of the International Physicochemical Standard of RhEPO CRS 1.0 using the capillary zone electrophoresis method in the quality control laboratories of both production facilities demonstrated an adequate performance of the analytical method described in the Ph. Eur., which was also demonstrated in previous studies by Vidal., et al. in 2024 [20]. These results constitutes the starting point for the development of the I-number and Ibio-number methods.
Obtaining I-number values very similar for CRS 1.0 in both facilities productive and the values reported by Hermentin in 2017 [11], constitutes evidence of the adequate performance of the method under the conditions analytical from both laboratories. The bioactivity obtained through the I-number assay for the CRS 1.0 using BRP 3 as standard in both laboratories, was very similar to each other and also in comparison with bioactivity certified for CRS 1.0 by rehearsal in mice [15]. The I- number values were determined with a good variability (˂ 1%) and bioactivity It was approximately 100% of the certified value, which is consistent with Hermentin's reports in 2017 [11].
Determining the CRS I - number value could constitute a tool useful for comparing the results of the verification of the ECZ compendial method for the identification of rhEPO in the different laboratories. The quality certificate of the CRS 1.0 standard shows he profile type of ECZ, but does not provide values certificates of the percentage of each of the eight isoforms of the profile, only the compliance with the broad ranks specified for each. The I-number of the CRS in change, constitutes a unique worth numeric that could allow compare the variability of the quantification of the percentage of isoforms between the different laboratories.
On the other hand, the I- number value, plus beyond allowing the determination of the activity rhEPO biological could be used like a good protein stability indicator since it reflects the structure of the molecule, and therefore, its sialylation and glycosylation. With the Establishing an I-number acceptance range, the broad criteria used by Ph. Eur. for identification through ECZ, they could be replaced by a range unique and quite narrow; which would provide an increase significant in the precision and accuracy of the test with an impact straight in the safety and efficacy of the drug.
The activity biological CRS 1.0 determined by Ibio-number method was very similar in both facilities productive and were obtained values very close to those reported by others authors [12,18]. The bioactivity was determined with a variability of 0.7% and with an accuracy Regarding power determined by bioassay close to 99%; which agrees with the results of others laboratories [12,18]. Ibio 's essay -number of the same way that the I -number test, allows calculate with good precision and accuracy of erythropoietin bioactivity.
These result constitutes evidence of adequate method performance in both laboratories, demonstrating that these methods are reproducible and together with the results of the I-number method; they are the starting point for the evaluation of manufactured rhEPO IFA batches on both productive facilities.
In the evaluation of the batches produced in the different production plants it was found that they complied with the specification of the Ph. Eur. for the rhEPO potency test (≥ 100,000 IU/mg of protein) using the three methods used [2]. The accuracy of the measurement by both methods was within the range described by different authors for the conventional method in mice (70-130%) [3,15,21]. In general, the I -number and Ibio-number physicochemical methods overestimated the potency with respect to the value obtained by in vivo, in a more marked in facility 2. This could be associated with the wide variability and little accuracy of tests biological in comparison with physicochemical methods according to their nature. However, in all cases the value estimated was found within the power range certain through the in vivo method.
According to the results presented, the use of the I-number and Ibio-number methods is only considered suitable for estimating the biological activity of rhEPO in batches that meet the established identity specifications using the ECZ method, the result of which constitutes the starting point for its application.
The performance of the compendial capillary zone electrophoresis method for the identification of erythropoietin under the experimental conditions of the laboratories of both production facilities of the Center for Molecular Immunology was verified. The I-number and Ibio-number methods were developed in both production facilities for the determination of the bioactivity of rhEPO with adequate precision and accuracy. The I-number method could constitute a useful tool to compare the quantification of the % of the isoforms of the international physical-chemical standard CRS between different laboratories and for the study of protein stability. It was demonstrated that the I -number and Ibio-number physicochemical methods are adequate to determine the bioactivity of the Active Pharmaceutical Ingredient of erythropoietin alpha; therefore, they constitute alternatives for the replacement of the in vivo method in normocythemic mice for the quality control of the product.
It is declared that there is no conflict of interest.
Copyright: © 2024 Danais Vidal Rossell and Jani Laffitte García This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.