Dr. Sundaram R, Dr. Srilakshmi Aluri, Vadiraj GB*, Aishwarya Raju, Vinay BS, Bineesh Eranimose and Neethumol Benny
R&D Centre, Ingex Botanicals Pvt Ltd, Nelamangala, Bangalore, Karnataka, India
*Corresponding Author: Vadiraj GB, Formulation Scientist, R&D Centre, Ingex Botanicals Pvt Ltd, Nelamangala, Bangalore, Karnataka, India.
Received: September 26, 2024; Published: September 28, 2024
Citation: Vadiraj GB., et al. “Identification and Quantification of Trace Levels of Acetone in Cultivars of Marigold Flowers and Oleoresin Using Gas Chromatography Coupled with Mass Spectrometry". Acta Scientific Pharmaceutical Sciences 8.10 (2024):28-34.
Acetone is one of the metabolites of fat breakdown in the human body as well as plants. Even synthesis of acetone during various microbiological fermentation reactions are well documented. Though acetone is one of the class III solvents with defined exposure levels, its occurrence naturally in some plants may pose challenge to chemists while controlling the quality of natural derived products like botanical extracts. In the current study, we report the presence of acetone in dried red and yellow marigold flowers, as well as their oleoresin. A Gas Chromatograph with MS/MS detector was used in Single Ion Monitoring (SIM) mode of analysis to detect and quantify acetone in dried red and yellow marigold flowers and their oleoresins. The study revealed that acetone is naturally present in both yellow and red varieties of marigold flowers as well as their oleoresins. This interesting finding also emphasize the need for careful monitoring and the quality control of marigold based ingredients and products. Additionally, it underscores the necessity of testing dried raw materials while studying the residual solvents in herbal ingredients and formulations to understand the source of residual solvents. Overall, this research contributes to better standardization in the herbal and phytochemical industries.
Keywords: Acetone Quantification; Yellow and Red Marigold Flowers; Oleoresin; Single Ion Monitoring Analysis
Acetone is an essential solvent in industry and an important starting point for organic synthesis. Nowadays, the most common way to create acetone is as a co-product of the very efficient and inexpensive phenol synthesis process from cumene. Petrochemical approaches, on the other hand, rely on non-renewable fossil fuels and are energy-intensive processes [1]. The sustainability of resources and their influence on the environment have been demonstrated to be benefits of bio-based businesses over traditional chemical industries based on fossil fuels. Although there are still certain limitations in this fermentation process, the acetone-butanol-ethanol (ABE) fermentation is a traditional biological method of producing acetone. Grains, maize, molasses, and other food-based feedstocks usually serve as substrates for the solvent-producing strains (such as Clostridium strains) in traditional ABE fermentation. Numerous metabolic engineering strategies were used in an attempt to increase the butanol output and ratio during the ABE fermentation [2,3]. However, certain enhancements have been done to improve product specificity. The modified strain of E. coli that was initially subjected to the acetone production pathway of C. acetobutylicum ATCC 824 accumulated 40 mM acetone [4] in a shake-flask culture using glucose as a carbon source. One thioesterase was substituted for the CoA-transferase of the acetone-synthesis cluster, enabling the pathway to function without the presence of acetate or butyrate. This resulted in a batch culture fed glucose to accumulate 122 mM acetone [5]. Phosphoketolase from Bifidobacterium adolescentis was also inserted into the genome of E. coli to create a non-oxidative glycolysis pathway. The production of 47 mM acetone from glucose in shake flasks was made possible by this, increasing the potential acetone output from 1 to 1.5 mol acetone/mol glucose [6]. The acetone synthesis from glucose by modified E. coli was found to have a higher titer and theoretical yield in these studies. It’s interesting to note that not only the microbes but even higher plants and humans synthesize acetone during their fat metabolism. In one of the studies, Tagetes minuta was reported contain acetone in the essential oil along with many other terpenoids [7].
Considering that Tagetes erecta is one of the major species of Marigold with immense medicinal benefits, there is an interest to study minor secondary metabolites like acetone in To date, there has been no comprehensive study on the quantification of acetone in dried red and yellow marigold flowers and marigold oleoresin. This research is the first to systematically investigate acetone levels in these specific samples. Given the limited data available in the literature, our study aims to address this gap and provide essential insights. The results will contribute to a better understanding of marigold constituents and their potential applications.
A variety of ailments have been treated with Tagetes erecta L. in history. The leaves' juice has been used to treat earaches, and the plant's leaves and florets are an emmenagogue. The infusion of the plant has been used as a vermifuge, diuretic, and carminative; it has also been used to treat rheumatism, colds, and bronchitis [8]. Its florets have been used as a laxative, and an extract from the roots has been used to treat ulcers and eye disorders [9]. Antimalarial and febrifuge properties have long been associated with the infusions of Tagetes erecta and Tagetes patula leaves [10]. Tagetes minuta has a potent larvicidal activity and has been traditionally used in Kenya [11] to repel mosquitoes. Its juice irritates the skin and eyes, while its flowers have stomachic, aperient, diuretic, and diaphoretic properties. Tagetes patula is a plant with stimulating and anthelmintic flower heads, leaves that contain particular principles that irritate skin, and roots and seeds that are used as purgatives. Tagetes patula flowers are used to make a carminative decoction, and their juice, which contains iodine, is applied to wounds and cuts. Tagetes lucida is a culinary plant that is used in soups instead of tarragon, and its leaves and flower heads are used to perfume bath water.
Tagetes erecta flowers were used to extract an essential oil that contained d-limonene, ocimene, 1-linalyl acetate, 1-linalool, tagetone, and n-nonylaldehyde [12]. Additional research found that the essential oil of Tagetes erecta included the following compounds: aromadendrene, phenylethyl alcohol, salicylaldehyde, phenylacetaldehyde, 2-hexen-1-al, eudesmol, tagetone, ocimene, linalyl acetate, and an unidentified carbonyl compound [13]. The essential oil of Tagetes erecta flowers was found to contain three acyclic monoterpene ketones, specifically 3, 7-dimethyloct-1-en-6-one, 3, 7-dimethyl-5-hydroxyoct-1-en-6-one, and 3, 7-dimethyloct-1, dien-6-one [14].
A standard stock solution was prepared by dissolving about 13.7 mg of acetone in a 10 mL volumetric flask, which initially contained about 5 mL of dimethyl sulfoxide (DMSO). Additional DMSO was added to reach the final volume, and the mixture was stirred well.
0.73 mL of the standard acetone stock solution was transferred into a 10 mL volumetric flask that already contained about 5 mL of dimethyl sulfoxide (DMSO). The solution was made up to the volume with additional DMSO and mixed well.
1.0 mL of the standard acetone stock solution was added to a 10 mL volumetric flask that previously contained about 5 mL of dimethyl sulfoxide (DMSO). The volume was then filled up to the level with more DMSO, and the solution was mixed well.
To prepare a 10 ppm solution from the acetone standard stock solution, 0.6 mL of the standard was diluted in a headspace vial containing 0.4 mL of dimethyl sulfoxide (DMSO). Then, 5 mL of water was added, the vial was sealed with a stopper, and the mixture was shaken well.
60 µL of acetone standard stock 2 was added to a 20 mL headspace vial containing 940 µL of dimethyl sulfoxide (DMSO). Then, 5 mL of water was added, the vial was vortexed, sealed with a stopper, and mixed thoroughly.
150 µL of acetone standard stock 2 was added to a 20 mL headspace vial that contained 850 µL of dimethyl sulfoxide (DMSO). Next, 5 mL of water was added; the vial was vortexed, sealed with a stopper, and mixed well.
300 µL of acetone standard stock 2 was added to a 20 mL headspace vial that contained 700 µL of dimethyl sulfoxide (DMSO). Then, 5 mL of water was added, the vial was vortexed, sealed with a stopper, and mixed thoroughly.
60 µL of acetone standard stock 1 was added to a 20 mL headspace vial that contained 940 µL of dimethyl sulfoxide (DMSO). Then, 5 mL of water was added, the vial was vortexed, sealed with a stopper, and mixed well.
150 µL of acetone standard stock 1 was added to a 20 mL headspace vial that contained 850 µL of dimethyl sulfoxide (DMSO) in it. Next, 5 mL of water was added, the vial was vortexed, sealed with a stopper, and mixed thoroughly.
300 µL of acetone standard stock 1 was added to a 20 mL headspace vial that contained 700 µL of dimethyl sulfoxide (DMSO). Then, 5 mL of water was added, the vial was vortexed, sealed with a stopper, and mixed well.
450 µL of acetone standard stock 1 was added to a 20 mL headspace vial that contained 550 µL of dimethyl sulfoxide (DMSO) in it. Then, 5 mL of water was added, the vial was vortexed, sealed with a stopper, and mixed well.
600 µL of acetone standard stock 1 was added to a 20 mL headspace vial that contained 400 µL of dimethyl sulfoxide (DMSO). Then, 5 mL of water was added, the vial was vortexed, sealed with a stopper, and mixed thoroughly.
A blank was prepared by adding 1 mL of DMSO to a suitable headspace vial containing 5.0 mL of water. The vial was then sealed with a stopper and mixed well.
500 mg of the sample was accurately weighed and placed into a 20 mL headspace vial. Then, 1 mL of DMSO was added to the vial containing 5.0 mL of water. The mixture was vortexed, sealed with a stopper, and mixed thoroughly. The sample was injected into the GCHSMS system, where it was processed for linearity and calculated using the formula provided below.
Calculate the amount of residual
Solvent in the sample (ppm) = Sample Area X Standard Concentration X Standard Purity X 10000
Standard Area X Sample Concentration
The acetone levels in dried yellow flowers remained stable, showing no significant variation due to the drying method used. These yellow flowers had slightly higher acetone concentrations than the red flowers, which may be attributed to their richer color and unique chemical compounds. Among all the samples tested, the oleoresin had the highest acetone concentration, likely resulting from the concentration of various metabolites during extraction. Overall, these results indicate that marigold oleoresin contains a greater level of acetone compared to both the dried extracts of red and yellow flowers Tables 1-3 and Figures 1-4.
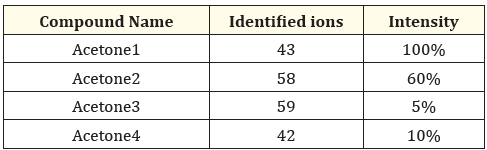
Table 1: MS1 scan of standard acetone (full scan).

Table 2: Most abundant mass for SIM (Single Ion Monitoring) mode of Analysis.
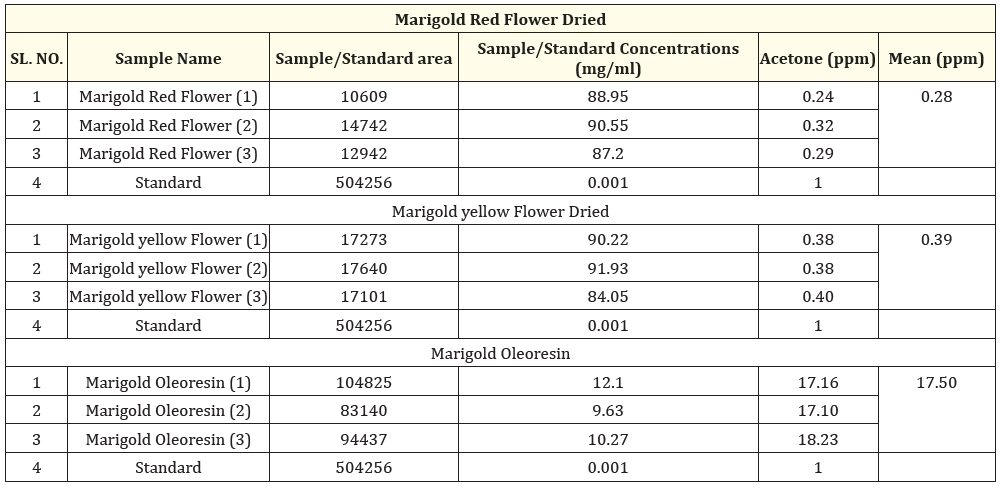
Table 3: Comparison of acetone from Marigold Red and Yellow flower dried and Oleoresin.

Figure 1

Figure 2

Figure 3

Figure 4
Acetone occurs naturally in plants, trees, volcanic gases, forest fires and as a product of the breakdown of body fat [15]. Acetone is one of the secondary metabolites produced in human body which is produced during the fat metabolism. Number of studies have been performed to quantify blood acetone levels as well as the acetone levels of the breath [16]. Considering its natural presence in plants and animals, we have investigated freshly collected and dried marigold flowers as well as the Oleoresin made from the dried marigold flowers. The results from the Single Ion Monitoring (SIM) analysis revealed that acetone is present in both dried yellow and red marigold flowers, as well as in the oleoresin extracted from marigold flowers. Its detection indicates that marigold flowers might contain volatile compounds that could be released during drying and extraction.
In the dried yellow flowers, acetone levels were stable, showing consistent concentrations regardless of the drying method used. However, the yellow flowers had slightly elevated acetone levels, as compared with red flowers, which could be related to their richer color and specific chemical compounds. The oleoresin had the highest concentration of acetone, likely due to the concentration of various metabolites during extraction.
The presence of acetone in these samples is important, as Marigold contains lot of fatty acids and esters of fatty acids with carotenoids. However, detection of acetone in Marigold confirms the fact that acetone is one of the secondary metabolites of living beings. This finding also emphasizes the necessity for careful monitoring of solvent residues in herbal ingredients which may give false positive results for acetone in natural products, though they are not intentionally used during the extraction process. Overall, this study highlights the need to understand the importance of acetone from marigold flowers and their extracts it needs further in-depth research to understand the biochemical pathways of acetone formation in Marigold flowers.
In conclusion, acetone is naturally present in various sources, including plants, trees, and as a by-product of fat metabolism in both humans and animals. Our findings indicate that Marigold flowers exhibit the presence of acetone and yellow flowers contain slightly elevated levels of acetone when compared to red flowers, potentially due to their distinctive chemical compositions. Due to the presence of acetone in the starting material, marigold oleoresin also demonstrated significant levels of acetone among the samples analyzed, suggesting a significant enrichment of metabolites during the extraction process. These results emphasize that while analysing the natural products, enough attention should be paid for the natural occurrence of solvents such as acetone in herbal ingredients, extracts and formulations. Overall, this research underscores the importance of understanding acetone concentrations in various botanical sources for future industrial applications.
The authors have no conflict of interest regarding the publication of this paper.
Copyright: © 2024 Vadiraj GB.,et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.