Mukthinuthalapati Mathrusri Annapurna* and Anga Swapana
GITAM School of Pharmacy, GITAM (Deemed to be University), Visakhapatnam, India
*Corresponding Author: Mukthinuthalapati Mathrusri Annapurna, GITAM School of Pharmacy, GITAM (Deemed to be University), Visakhapatnam, India.
Received: September 24, 2024; Published: September 28, 2024
Citation: Mukthinuthalapati Mathrusri Annapurna and Anga Swapana . “Analytical Techniques for the Assay of Clofarabine: A Review". Acta Scientific Pharmaceutical Sciences 8.10 (2024):26-27.
Clofarabine is an antineoplastic drug which acts by inhibiting the DNA synthesis and ribonucleotide reductase. In the present study the authors have summarised the analytical methods so far published for the estimation of Clofarabine in the literature.
Keywords: Clofarabine; Food and Drug Administration (FDA)
Clofarabine is an anti-cancer drug [1,2]. In 2004, the Food and Drug Administration (FDA) has approved. Chemically it is 5-(6-amino-2-chloro-purin-9-yl) -4-fluoro-2- (hydroxymethyl) oxolan-3-ol with molecular formula C10H11ClFN5O3 and molecular weight 303.68 grams/mole. Clofarabine (CAS no. 123318-82-1) converts in to its active triphosphate form and thereby competes with adenine triphosphate for use by DNA polymerase and finally leads to DNA damage that can trigger apoptotic pathways. Clofarabine (Figure 1) is used to treat leukemia in patients 1 to 21 years old.
Figure 1: Structure of Clofarabine (C10H11ClFN5O3).
Literature survey reveals that Clofarabine was studied by different analytical methods such as LC-MS/MS [3-5] HPTLC [6], HPLC [7-11] and UPLC [12] were developed for the estimation of Clofarabine and its impurities in pharmaceutical formulations and biological fluids. The earlier reported methods were summarized in Table 1.
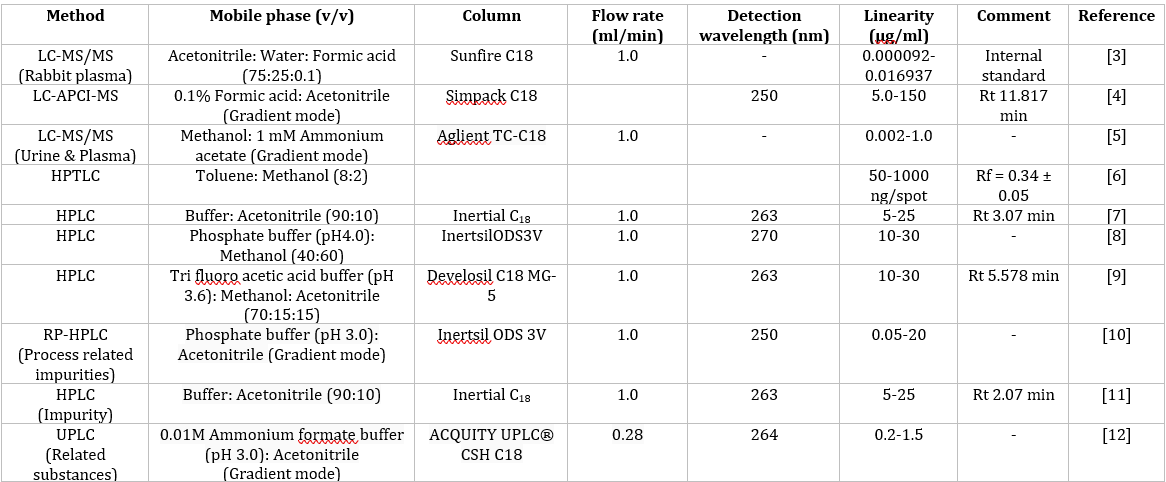
Table 1: Review of Clofarabine.
This review article explains different analytical methods as well as techniques developed for the estimation of Clofarabine in pharmaceutical formulations as well as body fluids.
Copyright: © 2024 Mukthinuthalapati Mathrusri Annapurna and Anga Swapana. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.