Karanam Sriharshitha*
Clinical Research Professional, Master’s in Clinical Research from the University of Mysore, India
*Corresponding Author: Karanam Sriharshitha, Clinical Research Professional, Master’s in Clinical Research from the University of Mysore, India.
Received:September 03, 2024; Published: September 16, 2024
Citation: Karanam Sriharshitha. “The Synergy of Real-World Evidence and Clinical Trials". Acta Scientific Pharmaceutical Sciences 8.10 (2024):16-21.
Randomized clinical trials (RCTs) are initial studies conducted to establish the safety and efficacy of an investigational product whereas Real-world evidence (RWE) is clinical evidence about the usage and potential benefits or risks of a medical product derived from analysis of RWD. The integration of Real-World Evidence (RWE) with traditional clinical trials represents a change in basic assumptions in the landscape of medical research and drug development. By bridging the gap between traditional trials and real-world applications, this collaboration not only accelerates innovation but also ensures that healthcare solutions are both effective and applicable in everyday clinical practice. The paper highlights the different methods and processes involved in RWE and clinical trials. A detailed comparison of RWE and clinical trials was listed. This constructive collaboration offers a comprehensive view of treatment effectiveness and safety. The review synthesizes recent research demonstrating how RWE can refine clinical trial designs, support regulatory decisions, and improve post-marketing surveillance. The review paper underscores the synergistic potential of combining RWE with clinical trials to enhance clinical research outcomes and the transformative impact of RWE in augmenting clinical trials, paving the way for more personalized, efficient, patient-centric drug development processes.
Keywords: Real-world Evidence (RWE); Clinical Trials (CT); Pre-Clinical Trials; Post-Marketing Surveillance (PMS); Electronic Health Records (EHR); Real World Data (RWD)
The use of Real-world evidence in drug and medical device regulations began in 2017. Real-world evidence (RWE) has emerged as a transformative force in drug development, complementing traditional clinical trial data and offering insights that enhance the development process and patient care [1]. RWE leverages data from everyday healthcare settings such as electronic health records (EHRs), insurance claims, patient registries, and wearables to comprehensively understand how treatments work in diverse, real-world populations. This review synthesizes recent research on RWE and its impact on drug development and patient care.
In evolving medical research, the dependency between Real-World Evidence (RWE) and clinical trials is emerging as a powerful combination for advancing drug development and patient care [2,3]. Traditionally, clinical trials have been the cornerstone of evidence generation, providing controlled, rigorous evaluations of therapeutic interventions. However, these trials often operate within tightly controlled settings that may not fully reflect the complexities and variabilities of everyday clinical practice.
By integrating RWE with clinical trial data, researchers can bridge the gap between controlled experimental conditions and real-world scenarios, yielding a more holistic understanding of a drug’s efficacy, safety, and overall impact [4,5].
This review aims to explain the dependency of RWE with clinical trials, examining how this integration enhances the design, execution, and interpretation of clinical research. We will explore recent advancements and case studies that highlight the potential of combining these two approaches, focusing on their collaborative benefits in refining trial methodologies, informing regulatory decisions, and improving post-marketing surveillance. Additionally, the review will address the challenges inherent in the fusion of these data sources, including data quality, consistency, and methodological rigor, while proposing strategies to overcome these obstacles. By explaining the interplay between RWE and clinical trials, this review underscores the transformative potential of this synergy in shaping the future of drug development and optimizing patient outcomes.
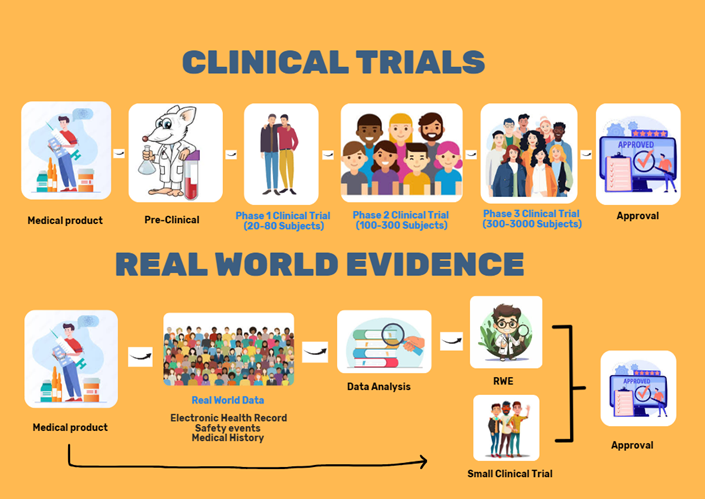
Figure 1: Overview of RCT and RWE.
The involvement of stakeholders in clinical research and real-world evidence studies is critical for ensuring the success and relevance of research efforts. Each stakeholder brings unique expertise and resources that contribute to the overall quality and applicability of the research findings [7]. By fostering collaboration and clear communication among stakeholders, researchers can enhance the impact of their work and advance the field of medicine and healthcare. Understanding these roles and interactions is essential for optimizing research processes and achieving meaningful outcomes that benefit patients and the healthcare system [1,6].
Parties involved in Clinical trials [8] are Regulatory authorities, like the FDA and EMA, who provide oversight and approval for study protocols and interventions. They ensure that the research complies with regulatory standards and that the interventions are safe and effective. Ethics committees, also known as Institutional Review Boards (IRBs), are responsible for reviewing and approving study protocols to ensure they meet ethical standards. They assess the risks and benefits of the research, ensuring that participant rights and welfare are protected throughout the study. Sponsors, often pharmaceutical companies, or research institutions, provide the funding and resources necessary for clinical research. They also oversee compliance with regulatory requirements and monitor the study's progress. CRO provides outsourced research services to pharmaceutical companies (Sponsors), biotechnology, and medical device companies. These services encompass various aspects of clinical trials, including design, management, and execution, as well as regulatory compliance and data analysis. Study site is a designated location where clinical research activities take place. It is typically a medical facility, such as a hospital, clinic, or private practice, where researchers conduct the study according to the trial’s protocol. Principal Investigators are responsible for designing, leading, and overseeing the research study. They ensure proper participant recruitment, informed consent, and adherence to the study protocol. Subject is an individual participant enrolled in the study according to the study protocol.
In Real-World Evidence, Regulatory bodies use RWE to assess the post-market safety and efficacy of interventions and to provide guidelines on the use of RWE in regulatory decisions. Healthcare providers will provide and collect patient data, which is essential for understanding treatment outcomes and patient experiences in real-world settings. Their involvement ensures that RWE is grounded in actual clinical practice and reflects the true impact of interventions on diverse patient populations. Patient advocacy groups are organizations that represent the interests of patients with specific conditions or health concerns. They work to improve patient outcomes, support research, and influence healthcare policies. Academic researchers are scholars who focus on generating and analyzing evidence derived from real-world data to inform healthcare decisions, policies, and practices. Health Technology Assessment (HTA) Agencies are organizations or bodies that systematically evaluate the properties and impacts of health technologies and interventions. Their goal is to provide evidence-based recommendations on the use, funding, and reimbursement of these technologies within healthcare systems. Funding agencies are Payers, including insurance companies, and health plans. Sometimes sponsor companies also provide funds to academic researchers to conduct RWE trials and companies will utilize the RWE data for their clinical trial and product launch [9-10].
RWE can provide insights into disease mechanisms and treatment responses that are not always apparent in traditional preclinical models. For example, Garcia et al. (2023) utilized EHR data to identify new therapeutic targets for cardiovascular disease, which were then validated in preclinical models. This approach ensures that targets are relevant to real-world patient populations. One of the biomarker discoveries has been Integrating RWE into preclinical research can aid in discovering and validating biomarkers [17]. Lee et al. (2022) demonstrated how real-world data from patient registries helped identify biomarkers for a new cancer therapy, which were then incorporated into preclinical studies to enhance model relevance and predict patient responses [18]. As an example of optimizing experimental design Chen et al. (2024) used real-world data to inform the design of preclinical diabetes models, resulting in models that better mimic human disease and treatment outcomes. RWE can guide the development of more relevant preclinical models by reflecting real-world disease variability and treatment patterns [19].
RWE can enhance the design of Phase I trials by providing insights into disease prevalence, natural history, and patient demographics. Research by Smith et al. (2023) utilized EHR data to refine the design of a Phase I oncology trial, identifying patient subgroups that might benefit most from the new therapy and tailoring the study design to include these groups. Real-world data can help determine more relevant starting doses and dosing regimens for early-phase studies [20]. For example, Johnson et al. (2022) analyzed real-world dosing patterns from insurance claims data to guide dose selection in a Phase I trial of a new antihypertensive drug, improving the initial dosing strategy and reducing the risk of adverse events [21].
RWE can aid in identifying and recruiting suitable participants for Phase II trials. A study by Chen et al. (2024) demonstrated how leveraging patient registries and EHRs improved recruitment strategies for a Phase II diabetes study by identifying eligible patients more efficiently and ensuring a representative sample [19]. Integrating RWE into Phase II trials allows for more precise patient stratification based on real-world variables such as comorbidities and prior treatment responses. Research by Davis et al. (2023) used real-world data to stratify patients in a Phase II cancer trial, leading to more targeted and effective treatment regimens [22].
RWE can support the validation of trial endpoints by providing insights into how endpoints perform in real-world settings. Lee et al. (2023) utilized patient-reported outcomes from RWE to validate endpoints in a Phase III cardiovascular trial, ensuring that the endpoints were meaningful and relevant to patients’ daily lives [23]. By incorporating RWE, Phase III trials can better reflect diverse patient populations and real-world conditions. Research by Williams et al. (2024) demonstrated how integrating RWE from various healthcare settings into a Phase III trial improved the generalizability of trial results and enhanced the external validity of the findings [25].
RWE is invaluable for post-marketing surveillance, providing insights into long-term safety and effectiveness in a broader patient population. A study by Brown et al. (2024) utilized insurance claims and EHR data to monitor the long-term safety of a new drug, identifying rare adverse effects and confirming sustained efficacy. Post-marketing RWE helps assess how drugs perform in everyday clinical practice compared to controlled trial conditions [24]. Nguyen et al. (2023) analyzed real-world data to evaluate the impact of a new rheumatoid arthritis medication on patient outcomes, demonstrating its effectiveness and identifying areas for further improvement [26].

Figure 1: Benefits of RWE studies with RCT.
Despite its potential, integrating RWE into preclinical studies presents challenges, including data quality, consistency, and integration with traditional research methods. Future research should focus on developing standardized protocols for integrating RWE into preclinical research, improving data interoperability, and addressing ethical concerns related to data privacy [27]. The integration process can be complex initially and may require advanced analytical methods and interdisciplinary collaboration to understand the data's better usage. regulatory considerations for using RWE in clinical trials.
Though there are high benefits with the RWE and CT collaborative approach, this combination is new and has limitations in initiating, planning, and executing it as a process. Advancements in data analytics, digital health technologies, and collaborative research efforts increasingly facilitate the integration of RWE and clinical trials. Embracing this combination will lead to more personalized and effective healthcare solutions and greater efficiency in drug development and approval processes. RWE Can be used to enhance the subject recruitment strategy, integrating the RWE data into a clinical trial design. RWE and CT can be worked out together with some limitations.
Copyright: © 2024 Karanam Sriharshitha. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.