Bhupesh Dewan*, Siddheshwar Shinde and Shweta Kondekar
Medical Services, Zuventus Healthcare Limited, Mumbai, Maharashtra, India
*Corresponding Author: Bhupesh Dewan, Medical Services, Zuventus Healthcare Limited, Mumbai, Maharashtra, India.
Received: August 09, 2024; Published: September 30, 2024
Citation: Bhupesh Dewan., et al. “An Active Post Marketing Surveillance of a Fixed-Dose Combination of Paracetamol, Phenylephrine and Chlorpheniramine for Symptomatic Treatment of Common Cold in Adults". Acta Scientific Otolaryngology 6.10 (2024):52-59.
Background: The global burden of respiratory diseases in India has drawn attention due to their high prevalence, socioeconomic impact, and lack of effective prevention measures. Objective: This study aimed to evaluate the safety and efficacy of a fixed-dose combination (FDC) of Paracetamol, Phenylephrine and Chlorpheniramine tablet in adults with common cold.
Methods: This study was conducted at four different locations across India and involved the participation of 200 individuals exhibiting common cold symptoms of recent onset. They were administered Maxtra®P tablets [FDC of Paracetamol 500mg, Phenylephrine Hydrochloride 10mg, and Chlorpheniramine Maleate 4mg] every 4 to 6 hours for a duration of 5 days. The primary endpoint focused on assessing the safety and tolerability of the investigational drug. This evaluation relied on the identification of adverse events (AEs) experienced by the patients and global assessment reported by both the Investigator and the patients. Secondary endpoint, aimed at analyzing efficacy, the proportion of patients achieving complete resolution and a reduction in the severity of symptoms.
Results: Adverse events such as nausea and dizziness, mild in severity, were experienced by two patients. However, they were resolved without any sequelae and were found not related to the study drug. Patients and investigators rated the treatment as “good or excellent” in alleviating symptoms associated with common cold in 92.5% (n = 185) and 99.5% (n = 199) of adults respectively, suggesting a positive response. A comprehensive assessment of 11 symptoms of common cold was conducted to evaluate the efficacy of the study drug. Complete resolution from the symptoms was achieved in 75% of patients and a statistically significant (p< 0.0001) reduction was observed in individual symptom severity score on day 5 as compared to baseline. A notable reduction in TSS (Total Symptom Score) was demonstrated where mean TSS at baseline was 16.05 which was reduced to 0.41 at day 5 (mean diff: 15.64, CI: 14.61 to 16.67, p< 0.0001).
Conclusion: Paracetamol, Phenylephrine Hydrochloride and Chlorpheniramine Maleate FDC was well tolerated and efficacious in the symptomatic treatment of common cold in adults.
Keywords:Adults; Common Cold; Fixed Dose Combination; Chlorpheniramine; Paracetamol; Phenylephrine
AE: Adverse Event; FDC: Fixed Dose Combination; TSS: Total Symptom Score
The common cold is a benign, self-limited syndrome representing a group of diseases. It is a separate and distinct entity, distinguishable from influenza, bacterial pharyngitis, acute bronchitis, acute bacterial sinusitis, allergic rhinitis, and pertussis. The term "common cold" refers to a mild upper respiratory viral infection involving, to variable degrees, nasal congestion and discharge (rhinorrhea), sneezing, sore throat, cough, low-grade fever, headache, and malaise [1]. Although it is usually self-limiting for healthy individuals, complications like secondary bacterial infections and exacerbations of existing respiratory conditions can occur. Despite its generally benign nature, the common cold imposes a significant economic burden due to increased medical consultations, school and work absenteeism and subsequent loss of earnings. Contrary to its name, it can be caused by a variety of distinct viruses, making it a diverse illness [2].
The global burden of disease reports has accentuated a high burden of chronic respiratory diseases in India. In an earlier study from India it has been reported that over half of patients who visited primary care physicians across 880 cities and towns, did so for respiratory symptoms [3]. Due to the high prevalence, socioeconomic burden, and lack of effective prevention measures, acute respiratory viral infections like common cold require strong medical attention [4]. The multi-symptom nature of common cold and flu has been highlighted in a recent review where the timeline of the illness indicates that up to eight symptoms may occur over the same period. Therefore, it is reasonable to develop multi-symptom medicines to conveniently treat the symptom complex [5].
The symptomatic treatment of the common cold has been evaluated in Cochrane meta-analyses, results of which showed that monotherapy did not improve the symptoms [6]. Multi-symptom treatments that contain several medicines to treat several symptoms simultaneously have become popular with healthcare professionals. The consumers also like the multi-symptom treatments because they provide safe way of treating multiple symptoms with what is viewed as a single treatment [5]. Decongestants and combination antihistamine/decongestant/analgesic medications can limit cough, congestion, and other variety of symptoms in adults. Several studies have also demonstrated that a triple combination of antihistamine, decongestant and analgesic offers relief from multiple symptoms in adults, with common cold and allergic rhinitis [7-9].
Considering the benefits associated, an active post-marketing surveillance was conducted to demonstrate the safety and efficacy of Maxtra®P tablet (a fixed dose combination of Paracetamol 500mg, Phenylephrine Hydrochloride 10mg, and Chlorpheniramine Maleate 4mg) in adults and to offer an alternative therapy aimed at providing symptomatic relief from common cold.
The present study was a multi-centric, open label, active post marketing surveillance. It was conducted in four geographically distributed sites across India from February 2021 to November 2021. This study was carried out in accordance with the protocol and the requirements of New Drugs and Clinical Trial Rules (NDCT Rules), 2019, Central Drugs Standard Control Organization-Good Clinical Practices (CDSCO-GCP), the International Conference on Harmonization- Guidelines for Good Clinical Practice (ICH-GCP) E6 (R2) and Declaration of Helsinki. After receiving approval from the respective institutional ethics committees (IECs), the study was initiated at each of the study centers. The trial was registered with the Clinical Trial Registry of India under registration number CTRI/2021/02/031390.
The participants were enrolled based on the inclusion and exclusion criteria. They underwent a screening procedure to determine their eligibility to participate in the trial. A total of 200 adult patients of Asian Indian Origin with symptoms of common cold of recent onset for more than 6 hours and less than 72 hours were enrolled. The eligibility criteria for the patients to be enrolled in the study included the following: Patients between the age group of 18 to 60 years. Patients with symptoms of common cold of recent onset, for more than 6 hours and less than 72 hours were included in the study. All patients provided written informed consent to participate in the study. Patients were excluded from this study if they had a history of hypersensitivity to any of the ingredients of the formulation, active on a monoamine oxidase inhibitor (MAOI), or barbiturates treatment, administered an antihistamine, analgesic or decongestant 01 day prior to study enrollment, pregnant or breastfeeding women.
Eligible patients were screened and enrolled to receive Maxtra®P tablet, a fixed dose combination of Paracetamol 500 mg, Phenylephrine Hydrochloride 10 mg and Chlorpheniramine Maleate 4 mg manufactured by Zuventus Healthcare Limited. The medication was administered one tablet every 4 to 6 hours daily for 05 days.
All eligible patients were informed about the study and signed written consent was taken to participate in the study. They underwent thorough medical history assessment and clinical examination prior to enrollment in the study. Patients were provided with Maxtra®P Tablets and asked to take 1 tablet every 4-6 hours for 05 days. Follow up visit was scheduled at day 5 (visit 2) for study assessment.
The primary endpoint focused on drug safety which was evaluated by the occurrence of adverse events. All serious and non-serious adverse events (if reported any) were fully documented in the case report form and causality assessment was done. Adverse events reported were followed up at scheduled visit and treated if necessary by the investigator till resolution.
The secondary endpoint was the proportion of patients experiencing (a) reduction in severity of symptoms (b) complete resolution of symptoms. The severity of symptoms was assessed using a subjective rating of eleven respiratory tract symptoms such as runny nose, nasal congestion, sneezing, sore throat, hoarseness, cough, wheezing, difficulty in breathing, headache, fever, and malaise. The symptoms intensity (severity) were evaluated on a five-point (0 to 4) scale (Table 1) defined as: 0 = no symptom; 1 = mild; 2 = moderate; 3 = severe and 4 = very severe. Global assessment of treatment response by the patient and investigator was documented based upon the symptom improvement, overall well-being, and any adverse effects experienced. Both investigators and patients assessed the response to therapy on a scale of 0 to 3, with 0 indicating Poor, 1 indicating Satisfactory, 2 indicating Good, and 3 indicating Excellent response.
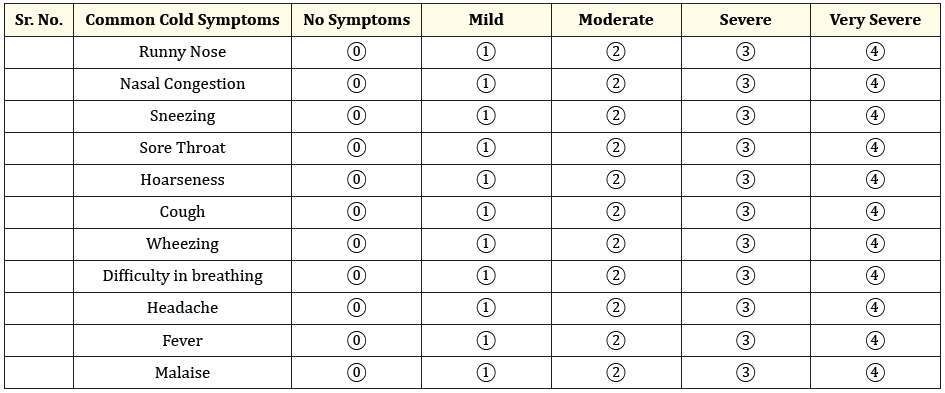
Table 1: Presentation of common cold symptom severity in the study.
0- no symptom; 1- mild; 2- moderate; 3- severe and 4- very severe, Assessed on baseline and day 5.
A total of 200 patients with symptoms of common cold of recent onset were enrolled and completed the study. The mean age of the population in this study was 38.5 years (range 18-60) years. Table 2 shows the demographic and baseline characteristics of patients enrolled in the study.
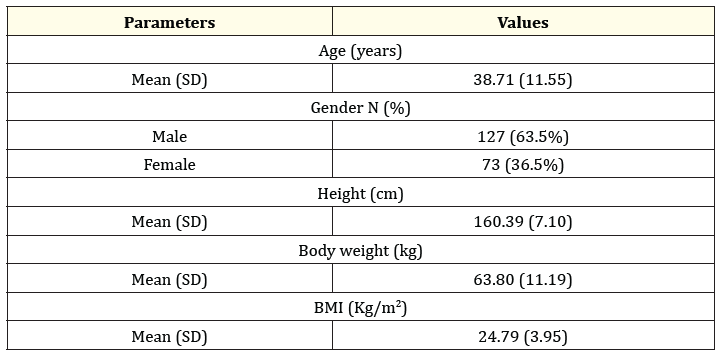
Table 2: Demographic data.
The primary endpoint focused on the safety of the study drug. Out of 200 patients, only 2 (1%) experienced nausea and dizziness. Causality assessment suggested that the AEs were mild in severity, not related to the study drug and resolved without any sequale. At the end of the study, the investigators and patients were asked to provide their response to therapy on a scale of zero to three, with zero indicating poor and three indicating excellent. Patients and investigators rated the treatment as “good to excellent” in alleviating symptoms associated with common cold in 92.5% (185/200) and 99.5% (199/200) of patients respectively, suggesting a positive response post treatment. The graphical representation of the same is made in Figure 1 and 2. Thus, it was concluded that Maxtra®P Tablet was found to be well tolerated.
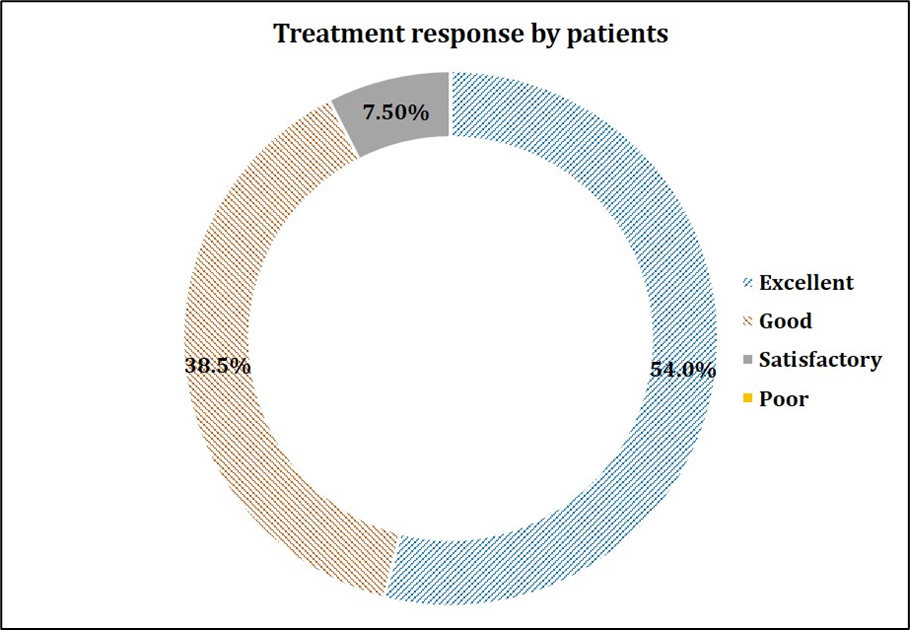
Figure 1: Global assessment of treatment response by the Patients.
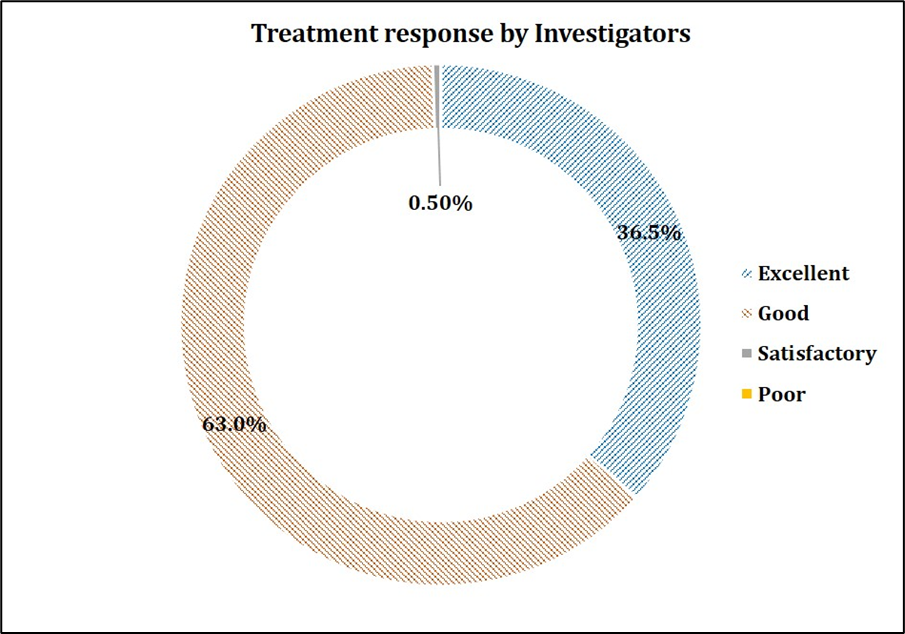
Figure 2: Global assessment of treatment response by the Investigators.
The secondary endpoint of the study analyzed the efficacy of the study drug. Assessment of the symptom score from baseline to Day 5 revealed that statistically significant (p < 0.0001) reduction in common cold symptoms was observed in the treatment group. This reduction in symptom severity score during the study duration is summarized in Figure 3. The TSS indicated a significant reduction in symptoms among the patients. Initially, the mean TSS was 16.05 and plummeted to 0.41 after 5 days of treatment. The remarkable statistically significant mean difference of 15.64 (95% CI: 14.61 to 16.67, p < 0.0001) highlighted the efficacy of the intervention.
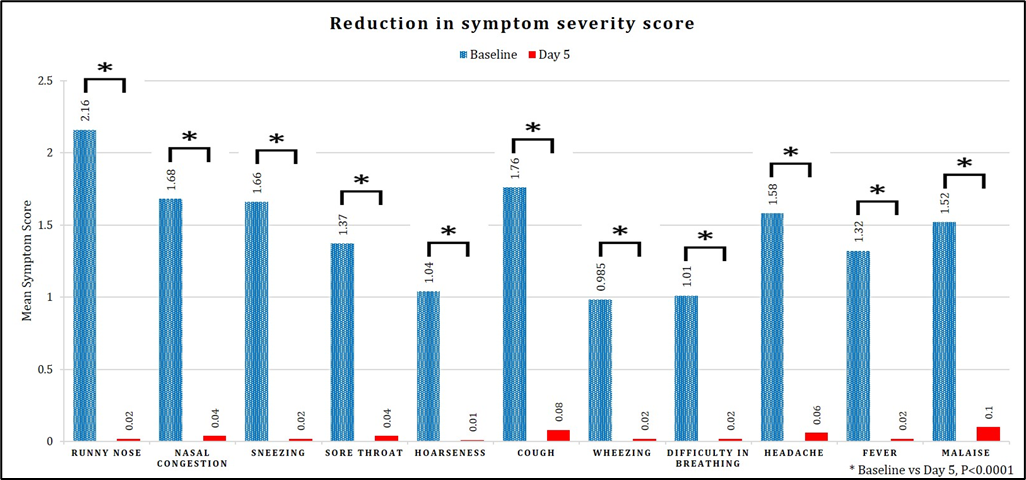
Figure 3: Assessment of symptom severity score from baseline to day 5.
Out of 200 enrolled patients, complete resolution from the symptoms was observed in 150 (75%) patients. Improvement in nasal, respiratory and generalized symptoms over the study period is presented in Figures 4, 5, 6. Graphical representation of the assessment of symptom severity depicts that majority of the patients demonstrated symptom relief wherein >98% were free from runny nose, sneezing, and hoarseness; and >95% did not experience wheezing, difficulty in breathing, fever and nasal congestion based on a pre-defined symptom severity scale. At the end of the study, none of the patients reported very severe symptoms, and there was no indication of worsening of their condition.
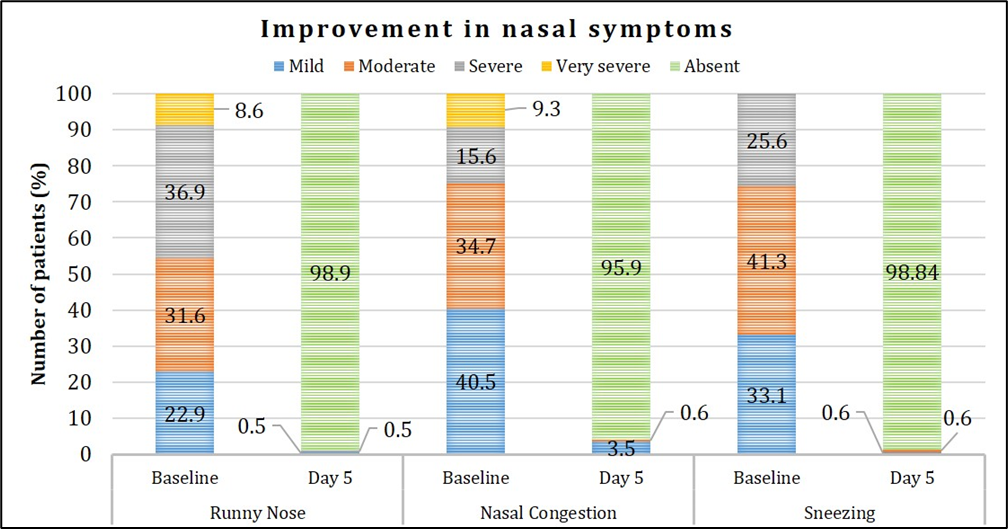
Figure 4: Assessment of improvement in nasal symptoms.
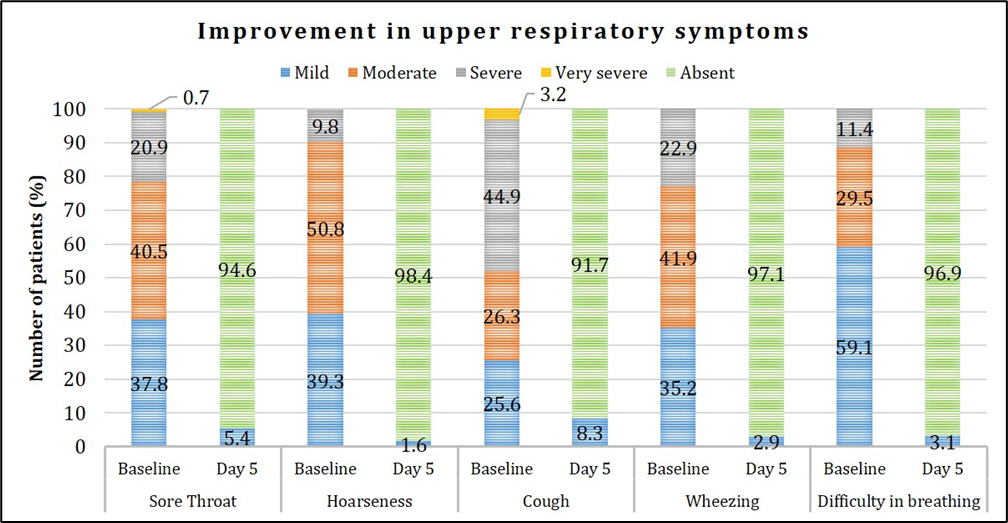
Figure 5: Assessment of improvement in upper respiratory symptoms.
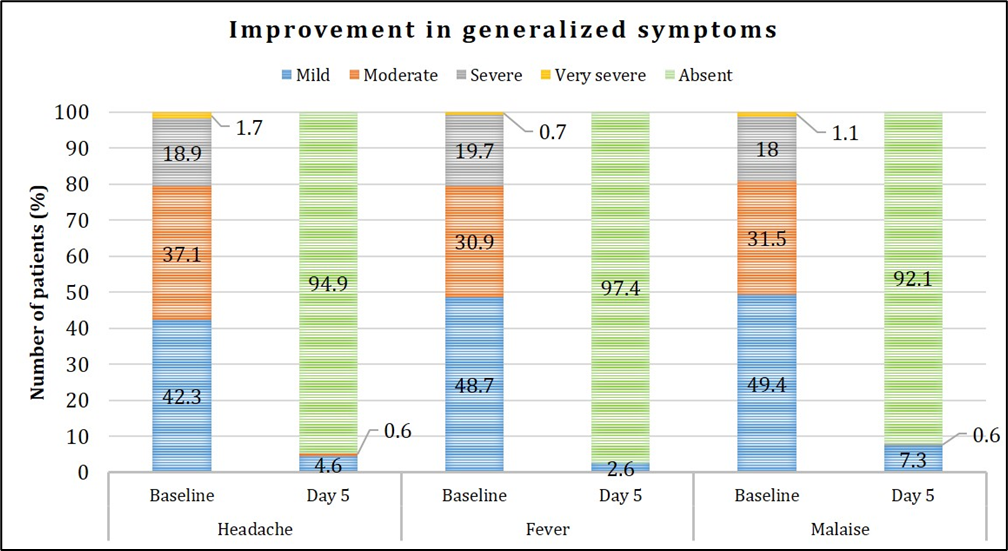
Figure 6: Assessment of improvement in generalized symptoms.
Colds are usually self-limiting to healthy individuals, but there are also recognized complications such as secondary bacterial infections, exacerbations of asthma, chronic obstructive airways disease and cystic fibrosis [10]. Beyond impairing the quality of life, common colds have a tremendous economic burden on societies due to work absenteeism. Thus, treatments that reduce the incidence of infection, lessen the severity of symptoms, shorten the duration of common cold are of high interest both for the individual and society [11]. Common cold is defined by its multi-symptom nature with evidence showing a variety of symptoms reported simultaneously each day over the first six-seven days of illness. Multi-ingredient combination for multi-symptom relief are therefore formulated to safely, simply, and simultaneously treat multiple symptoms associated. The rationale for the formulation of combination products is thus, practical, logical and reasonable [12].
According to Cochrane review [7], Picon PD., et al. [8] and Eccles R., et al. [12], a combination of analgesic, decongestant and antihistamine provides better relief for multiple symptoms in common cold. Maxtra®P is one such product which incorporates a blend of Paracetamol, Phenylephrine Hydrochloride, and Chlorpheniramine Maleate. This triple combination provides benefit in adults by treating varied symptoms associated with common cold. Antihistamines are commonly used in multi-symptom relief medicines as they have a broad pharmacological activity which make them particularly beneficial. Chlorpheniramine maleate has been shown to be effective and is widely used for treating common colds [12,13]. Paracetamol is used as the major ingredient in combination medications for the common cold. As a non-steroidal anti-inflammatory drug (NSAID), it exhibits antipyretic and analgesic effects [14]. Phenylephrine, an oral decongestant is marketed for relief from nasal congestion due to the common cold or other upper respiratory tract allergies [8]. An active post marketing surveillance study was thereby conducted to evaluate the safety and efficacy of Maxtra®P tablet in the Indian population.
In the present study, reduction in severity of symptoms and complete resolution was analyzed to demonstrate efficacy of the investigational drug in treating common cold. The comparison of baseline and day 5 scores revealed that patients experienced major reduction in symptoms associated with common cold. Post treatment with Maxtra®P tablet, majority of the patients exhibited symptomatic relief. More than 95% of the patients reported absence of symptoms based on a pre-defined symptom scale. This confirms the combination’s efficacy in providing symptomatic relief to the patients. The evaluation of symptom severity by the investigators led to the conclusion that a statistically significant (p < 0.0001) reduction was observed in the study group after the administration of study drug.
Evaluating the severity scores of the individual symptoms and the total symptom severity during this study highlighted the effectiveness of Maxtra®P tablet. In the present study, initially the TSS was 16.05 (baseline) which dropped dramatically to 0.41 (day 5) with a mean difference of 15.64. This substantial improvement indicated a remarkable 97% reduction in the TSS post-treatment. Kiran., et al. [9] in a phase IV clinical trial conducted on a similar combination, also reported that 89.6% reduction in TSS was observed within a span of five days. Several different studies [15-18] conducted by Kiran., et al. reported a consistent decrease of 97.6%, 91.4% 87.7% and 83.3% in TSS following the same duration of treatment. In a placebo controlled trial by Picon., et al. [8] it was revealed that the overall symptom score was reduced in both placebo and treatment group but a statistically significant difference (p = 0.015) was observed in between the groups. He emphasized on the fact that the difference observed provided evidence for the study drug’s efficacy rather than being solely due to a placebo effect.
The safety and efficacy of any treatment primarily depends on the patient's adherence to taking their medications as directed. It is evident that simpler dosing regimens lead to higher patient compliance [19]. FDCs can result in more consistent and effective management of symptoms, ultimately improving compliance and reducing the overall burden of illness. Moreover, it is reported that FDCs do not pose any additional safety risks compared to their respective single-ingredient products. Our study results were consistent with similar observations. Safety analysis showed that only 1% experienced mild adverse events. Additionally, published literature [8,9,15-18] has concluded that when administered as prescribed, combination therapy is efficacious and well tolerated. The study results thus revealed that Maxtra®P tablet provides symptomatic relief and can be used for the treatment of common cold in adults.
This post-marketing study sought to evaluate the safety and efficacy of combination product targeting the common cold symptoms. The study findings demonstrated that each of the components has its own distinctive contribution to the efficacy of the combination and is well tolerated in treating common cold.
We express deep gratitude to Dr. Sunil Naik (Government Medical College and General Hospital, Srikakulam), Dr. Manoj Gupta (Health Point Hospital, Kolkata), Dr. Prasita Kshirsagar (Rajiv Gandhi Medical College and Chhatrapati Shivaji Maharaj Hospital, Thane) and Dr. Manoj Pal (Janta Hospital, Varanasi).
The authors declare that they have no conflict of interest.
Copyright: © 2024 Bhupesh Dewan., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.