Ishwar Subedi1*, Rabindra Tuladhar2 and Shrijana Rai3
1Department of Food Technology and Quality Control, Kathmandu, Nepal
2Himalayan College of Agricultural Science and Technology, Purbanchal University, Nepal
3Food Import Export and Quality Certification Office, Kathmandu, Nepal
*Corresponding Author: Ishwar Subedi, Department of Food Technology and Quality Control, Kathmandu, Nepal.
Received: September 30, 2024; Published: October 27, 2024
Citation: Ishwar Subedi., et al. “Extension of Raw Milk Shelf Life Applying Lactoperoxidase System". Acta Scientific Nutritional Health 8.11 (2024):41-44.
Lactoperoxidase system (LP-S) in the raw milk samples were collected and activated by the addition of potassium thiocynate (KSCN) and hydrogen peroxide (H2O2). The 11 mg/ml of KSCN was added to make overall thiocyanate content 14mg/ml, as initial thiocyanate of milk was 3 mg/ml in an average. The collected milk sample was divided into four different parts. One part was left with no treatment with KSCN and H2O2. Other three parts of milk were treated with 0.02%, 0.04%, and 0.06% of H2O2. Physiochemical analysis of the milk was done. As per the analysis, initial acidity of normal milk was 0.14%, while the initial total plate count (TPC) for summer milk (without cold chain and with cold chain) were found to be 5.0 and 3.4 log cfu/ml, respectively. Similarly, initial acidity of normal milk was 0.13% and the TPC for winter milk samples were found to be 5.0 and 4.0 log cfu/ml, respectively. In the same way Coliform count of summer collected milk samples were found to be 4.13 and 2.1 log cfu/ml, respectively. Likewise, for winter collected milk were found to be 4.13 and 1.5 log cfu/ml respectively. The two ways ANOVA showed that the combination of the milking season and peroxide concentration had significant different association with the acidity (p < 0.05). Similarly, the effect of peroxide concentration and milking condition had significant different association on acidity (p < 0.05). Since three different H2O2 concentrations had same effect on the shelf-life of the milk, it was concluded that by adding 0.06% of H2O2, LP-S in raw milk was activated with maximum shelf-life of 18 hours.
Keywords: Acidity; Hydrogen Peroxide; Lactoperoxidase; TPC
Raw milk is a nutrient-rich liquid that provides an ideal environment for the growth and multiplication of microorganisms due to its composition of proteins, carbohydrates, fats, minerals, and vitamins. In regions where refrigeration is impractical, especially in tropical and subtropical climates, alternative methods for retarding bacterial growth during milk collection and transportation are essential. Among these, the activation of natural antibacterial enzymes in milk has proven effective. One such system is the Lactoperoxidase System (LP-S), which involves the enzyme lactoperoxidase in combination with hydrogen peroxide (H2O2) and thiocyanate [SCN]-. This system produces potent antibacterial effects, significantly inhibiting bacterial growth in raw milk. While the natural LP-S loses effectiveness within two hours of milking, it can be reactivated through the addition of thiocyanate and H2O2 [1]. The LP-S is especially valuable for farmers in rural areas lacking refrigeration, offering a cost-effective means to extend the shelf life of raw milk and other dairy products [2]. This system has both bactericidal and bacteriostatic effects on a variety of microorganisms. It is more effective against Gram-negative, catalase-positive bacteria (e.g., coliforms and Salmonella), which are killed, whereas Gram-positive bacteria are generally inhibited but not killed. LP-S operates optimally at acidic pH, though lactoperoxidase is less stable in such conditions [3].
The LP-S is widely used in raw milk preservation due to its effective antimicrobial properties. By activating the LP-S through the addition of H2O2 and [SCN]-, the shelf life of raw milk can be extended, even under non-refrigerated conditions [4]. For instance, adding 0.25 mM of H2O2 and [SCN]- can extend milk's shelf life at 10°C for up to three days. LP-S has also been used in yogurt production to control post-culturing acidification and in stabilizing pasteurized milk and emulsions. Substituting [SCN]- with iodine further extends raw milk’s shelf life to 10 days, showcasing the system's bactericidal effect [4]. In Nepal, most milk producers are rural farmers, where refrigeration is not economically viable, leading to milk spoilage during transportation to processing plants. Traditional methods like pasteurization, refrigeration, and chemical preservatives are either too costly or impractical for small-scale dairy production. With up to 80% of milk entering the informal market, there is a need for an affordable alternative to extend the milk supply chain. The LP-S offers a cost-effective solution for rural farmers, providing a means to preserve milk without expensive equipment, making it an ideal method for improving milk quality in remote areas. The research on the LP-S is vital for small dairy farmers in developing countries like Nepal, as it offers a cost-effective method for preserving raw milk without relying on expensive refrigeration [1,5]. LP-S can extend the shelf life of milk, reducing post-harvest losses and ensuring a more stable supply of safe milk for markets. This, in turn, boosts income generation, enhances household food security, and improves nutrition. By applying LP-S, smallholder farmers can increase their participation in global dairy markets, contributing to poverty alleviation, healthier communities, and sustainable dairy production in regions with limited resources. Here, the research is expected to extend the shelf life of raw milk using the LP-S. Specifically, it aims to identify the optimal H2O2 concentration for maximum preservation and determine the maximum shelf life (in hours) of raw milk after applying the system.
The microflora of raw milk is influenced by multiple factors, although milk produced in the mammary glands of healthy animals is initially sterile. Microorganisms enter the udder through the teat duct opening. Common bacteria include Gram-positive cocci, streptococci, staphylococci, micrococci, lacticacid bacteria (LAB), pseudomonas, and yeast [6]. Psychrotrophic bacteria such as Pseudomonas, Acinetobacter, Flavobacterium, and Bacillus can grow at low temperatures, while thermoduric genera like Microbacterium, Micrococcus, and Clostridium spores survive heat treatments. The udder’s mucosal membrane harbors a microflora that includes streptococci and staphylococci, with contamination level of 10² to 104 cfu/ml. Milk from animal with mastitis often shows clots, colour changes, or altered viscosity and should not be used for consumption. Sources of milk contamination include the interior and surfaces of the udder, milking equipment, the environment (air, water), and the handlers [7]. Proper hygiene practices are essential to minimize microbial contamination in milk.
Raw milk contains several inherent antimicrobial components that protect against microbial contamination. Immunoglobulins, particularly in colostrum, provide immediate immune defence to new-borns. Bovine milk has four types of immunoglobulins: IgG1, IgG2, IgM, and IgA, which help protect against pathogens. Phagocytosis, primarily by polymorphonuclear leukocytes (PMNs), is crucial for combating infections such as mastitis. Non-specific antimicrobial proteins include xanthine oxidase, lactoferrin, and lysozyme. Xanthine oxidase produces H2O2, contributing to milk's antimicrobial activity. Lactoferrin chelates iron, depriving bacteria of essential nutrients, while lysozyme disrupts bacterial cell walls. These components collectively enhance milk's resistance to microbial growth [8].
The lactoperoxidase System (LP-S) is a natural antimicrobial defense found in body fluids such as milk, saliva, tears, and gastric juice. In milk, lactoperoxidase, an enzyme found in concentrations of approximately 30 mg/l, plays a crucial role in protecting newborn animals by creating antimicrobial compounds when mixed with saliva [9].
Lactoperoxidase, in the presence of hydrogen peroxide (H₂O₂) and thiocyanate ions ([SCN]⁻), produces antibacterial compounds, primarily hypothiocyanite ([OSCN]⁻) [10]. This system exhibits:
These reactions inhibit bacterial metabolism, preventing spoilage and extending milk's shelf life, especially useful in warm climates where refrigeration may be limited [11,12].
The LP-S is activated by adding [SCN]⁻ (about 14 mg/l) to milk, followed by a controlled release of H₂O₂, typically from sodium percarbonate (Na₂H₃CO₆). The H₂O₂ disappears within five minutes, and the system remains effective, extending milk's shelf life by 7-8 hours at ambient temperatures [13]. The primary antimicrobial action occurs through the lactoperoxidase-catalyzed oxidation of [SCN]⁻ into [OSCN]⁻, which, at an optimal pH of 5.3, balances with hypothiocyanous acid (HOSCN), a more potent bactericide. [OSCN]⁻ interferes with bacterial glycolysis, disrupts NADH/NADPH-dependent reactions, and oxidizes bacterial sulphydryl groups, leading to the leakage of cellular components [14].
Studies have confirmed the safety of the LP-S. The [OSCN]⁻ produced has a short half-life, and its by-products are considered non-toxic. Joint evaluations by the WHO/FAO Expert Committee on Food Additives (JECFA) have deemed the LP-S to present no toxicological hazard, making it a safer alternative to using H₂O₂ alone, especially in regions without refrigeration. The LP-S is an effective, safe, and natural antimicrobial system that enhances milk preservation by targeting bacterial growth, without posing toxicological risks, even after 15 years of field testing [15]
The methodology for the LP-S study involved collecting milk samples from Holstein cows in the Kathmandu valley during both summer and winter seasons. The collected milk samples were divided into four parts. One part was left untreated, while the other three parts were treated with 0.02%, 0.04%, and 0.06% H2O2, along with the addition of KSCN (14 mg/l). Milk acidity was determined following the National Dairy Development Board (NDDB) protocol [16], where 4 mL of raw milk was mixed with 20% (w/v) trichloroacetic acid, left for 30 minutes, and filtered using Whatman No 40 filter paper. The 1.5 ml of the filtrate was mixed with the same volume of ferric nitrate reagent, and absorbance was measured at 460 nm within 10 minutes. Thiocyanate concentration was determined against a standard curve. Microbial load and coliform counts were evaluated to assess the efficacy of the LP-S. The data were analyzed using Analysis of Variance (ANOVA) in Gen Stat Discovery, with the mean differences separated using the Least Significant Difference (LSD) method at a 5% significance level [17].
The study focused on evaluating the LP-S in raw milk collected from Holstein cows. The physicochemical properties of both normal and cold chain maintained raw milk were analyzed, showing minor differences, especially in fat content. The LP-S was activated by adding KSCN H2O2, which resulted in a slower increase in acidity and microbial counts, extending the milk's shelf life. Among different H2O2 concentrations, 0.06% treatment proved most effective in preserving milk at higher temperatures. The LP-S was found to effectively slow microbial growth, maintaining better quality in milk under storage conditions.
In comparing raw milk acidity and shelf life at 35°C, LP-S activated milk samples showed lower acidity than control samples, indicating slow microbial growth due to LP-S's antimicrobial properties. The shelf life of normal control milk was 4 hours, while cold chain maintained collected control milk lasted 6 hours. LP-S treated normal milk matched cold collected control milk at 6 hours. LP-S activation reduced microbial counts, especially in collected cold milk. Coliform growth was slower in hygienically collected milk, aligning with previous studies.
The results showed that acidity increased over time in both control and LP-S treated milk, but the rate of increase was slower in LP-S activated samples, indicating better microbial control. Milk treated with 0.06% H2O2 had slightly lower acidity compared to 0.02% and 0.04% H2O2 treatments. The control sample showed a positive test for Clot on boiling (COB) test on fifth hour of storage, while for the LP-S activated milk, the COB test showed a positive result at the seventh hour after activation.The results showed that in control samples, the Total Plate Count (TPC) increased every hour. In LP-S activated samples, the TPC decreased in the first hour and remained nearly constant in the second hour, likely due to lactoperoxidase enzyme depletion. Among the H2O2 treatments, milk treated with 0.04% and 0.06% had lower TPC counts compared to 0.02% treated milk. The control sample tested positive for the COB test at the seventh hour, while LP-S activated milk treated with 0.04% and 0.06% H2O2 tested positive at the ninth hour and 18 hours longer respectively.
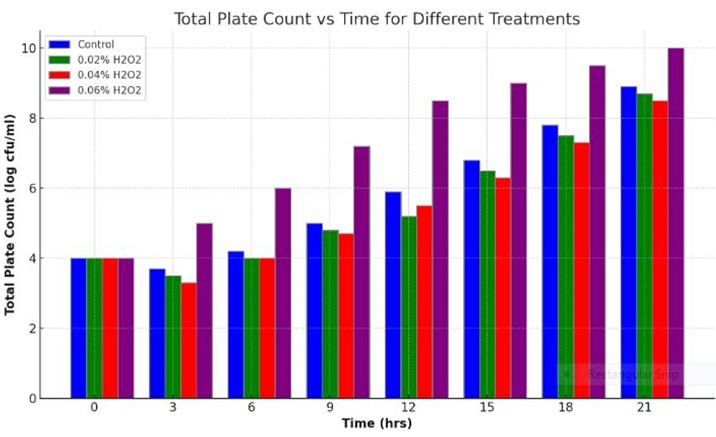
Bar Diagram
The bar diagram illustrating the Total Plate Count (log cfu/ml) over time for different treatments, including Control and various concentrations of hydrogen peroxide (H2O2) was correlated. The lower the acidity means also the lower TPC at 0.06% H2O2. Each set of bars represents the data at specific time points across the treatments where 0.06% has shown the highest keeping time of raw milk with lower TPC count.
The research on LP-S activation in milk was compared with microbial and acidity changes in summer and winter, measuring TPC, acidity, clot on boiling and coliform bacteria. In control samples, TPC steadily increased, with a rapid rise after the first hour, whereas LP-S treated milk showed a slower increase, demonstrating a bacteriostatic effect. Coliform counts in control samples grew steadily, while in LP-S activated samples, they initially decreased. Among treatments, 0.06% H2O2 was most effective, maintaining lower coliform counts. Acidity increased over time in all samples, but LP-S treated milk had a slower rate of acidity development, confirming its antimicrobial properties. The shelf life of LP-S treated milk was extended compared to control samples, especially in hygienically collected milk. The study supports LP-S activation that slows microbial growth and extends milk shelf life.
Copyright: © 2024 Ishwar Subedi.,et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.