María de los Ángeles Jofré1*, Laura S Favier1, Valeria A Cianchino1, Julia Divizia3, Javier E Ortiz2, Gabriela E Feresin2 and Claudia A Ortega 1
1Área de Tecnología Farmacéutica, Facultad de Química, Bioquímica y Farmacia, Universidad Nacional de
San Luis, Argentina
2Instituto de Biotecnología, Facultad de Ingeniería, Universidad Nacional de San Juan and CCT CONICET.
San Juan Av, Argentina
3Laboratorios Puntanos S.E. Av. del Fundador s/n, Puente Blanco, San Luis, Argentina
*Corresponding Author: María de los Ángeles Jofré, Área de Tecnología Farmacéutica, Facultad de Química, Bioquímica y Farmacia, Universidad Nacional de San Luis, Argentina.
Received: September 12, 2024; Published: September 24, 2024
Citation: María de los Ángeles Jofré., et al. “Design and Development of Soap Herbal Tablet of Larrea divaricata (Cav.) with Antifungal Activity Against Candida albicans". Acta Scientific Nutritional Health 8.10 (2024):42-48.
In this present work soap herbal tablet of Larrea divaricata Cav. ethanolic extract (LdE) with antifungal activity against Candida albicans, was designed and developed.
The chemical profile of main compounds and quantification of nordihydroguaiaretic acid (NDGA) in the LdE was performed by means of UPLC-MS/MS. Two formulations (F1 and F2) were prepared by direct compression with different concentrations of active herbal ingredient (AHI). The performance of three carriers for impregnating LdE was evaluated. TRI-CAFOS® 500 was selected as carrier after a comparative study by Scanning Electron Microscope with AEROSIL® 200 and AEROPERL® 300. StarLac® 90, a co-processed lactose, was employed as binder. The soap herbal tablets quality parameters were evaluated in accordance with United States Pharmacopeia (USP-43) guidelines, weight variation, hardness, friability, disintegration test, foam stability test and microbiological assay against Candida albicans were performed.
The F1 (LdE + TRI-CAFOS®500 (LdET1) 62.8%, StarLac®90 0.1%, Microcrystalline cellulose 10%, vitamin E 0.1%, Betan F® 5%, Lactic acid 5%, Rose essence 0.1%, Low density Hyaluronic acid 1.5% and magnesium stearate 0.5%) showed optimal rheological properties, good biopharmaceutical parameters (Disintegration test (27.33s), Hardness (3.00Kp), Friability (0.92%), Foam stability (1.12)) and Minimum inhibitory concentration (MIC) 25 mg/ml for Candida albicans. Considering that there are no soap herbal tablets on the market, the present design can be used as a novel herbal cosmetic form of vaginal hygiene.
Keywords: Larrea divaricata Cav. Extract; Soap Herbal Tablets, StarLac® 90; Candida albicans
Candida species as a part of the vaginal flora for unknown reasons change from a commensal organism to a pathogenic one causing vulvovaginal [1]. The most common etiological agent causing fungal inflammation is Candida albicans, a polymorphic opportunistic fungus, but infections caused by other strains are also possible.
Vulvovaginal candidiasis (VVC) is a common condition responsible for one-third of all cases of vulvovaginitis in women of reproductive age. Approximately 70% of women report having had this condition at some point in their lives, and an estimated 8% of women experience recurrent VVC.
Many factors can promote or even induce, such as local defense mechanism dysfunctions, gene polymorphisms, allergies, antibiotics, serum glucose levels, psychosocial stress, estrogens. The potential mechanisms favoring Candida vaginal colonization and the host factors enhancing the diversion to infection often raise the question of whether clinicians should administer treatment or not. The VVC is divided into uncomplicated and complicated cases. Uncomplicated ones are sporadic episodes of mild infections caused by C. albicans. Complicated cases are severe infections caused by non-albicans Candida species (C. glabrata, C. tropicalis, C. krusei, and C. parapsilosis), recurrent VVC, VVC during pregnancy, or VVC associated with other medical conditions such as immunosuppression or diabetes [2].
Larrea divaricata Cav. commonly known as “jarilla” and belonging to the Zygophyllaceae family, is a shrub native to west-central Argentina. It is recognized for its antioxidant properties and is traditionally used as an antiinflammatory agent or to treat fungal and bacterial infections [3]. L. divaricata dry extracts have demonstrated effectiveness against Candida species from vaginal infections, including both Candida albicans and non-albicans strains [4]. The chemical profile of L. divaricata extracts reveals nordihydroguaiaretic acid (NDGA) as the primary metabolite, and flavonoids as part of the profile of polyphenolic compounds [5].
This species is commonly utilized as immunomodulatory and antitumoral agent, for treating various ailments including sores and wounds, rheumatism, inflammation, gastric disturbance, venereal diseases, arthritis, tuberculosis and common cold. In previous works, Martino., et al. [6], demonstrated the antifungal activity and Stege., et al. [7], the antibacterial activity of different extracts of L. divaricata.
Conventional synthetic drugs such as imidazole related compounds or polyenic derivatives are used for the treatment of candidiasis [8]. Although are still the mainstream therapy for VVC, their therapeutic value is limited due to potential toxicity, the appearance of drug-resistant strains and high recurrence rates [9].
In recent years there has been a remarkable increase in the use of herbal medicines in health care. As part of conventional medicine, phytomedicine provides therapeutically effective extracts that can be embedded in dosage forms, such as, capsules, tablets. However, new drug delivery strategies are needed to improve the management of the treatment of common diseases and the treatment of vulvovaginal cavity diseases.
Medicinal plant extracts may contain hundreds or even thousands of individual bioactive compounds in varying abundances and identifying the bioactive compounds responsible for a given biological activity is a significant challenge [10]. The goal of herbal formulation development is to deliver synergistic, potent, agonistic/antagonistic pharmacological agents within themselves and work together in a dynamic manner to produce maximum therapeutic efficacy [11].
On the other hand, tablets are the most popular solid dosage forms [12]. Soap tablets are a convenient and environmentally friendly option for vaginal cavity hygiene. They come in small, dissolvable tablets that can be easily stored and transported. Soap tablets offer significant environmental benefits compared to traditional liquid soaps because no water is used in their preparation. In addition, compressed soap often contains fewer chemical additives and preservatives.
In the present work the design, development and evaluation of soap herbal tablets of LdE by direct compression as alternative treatment vaginal candidiasis we described.
Organic solvents, chemicals and reagents of analytical grade were purchased from Sigma-Aldrich. The direct compression excipients investigated in this study included: Aeroperl® 300 Pharma, TRI-CAFOS®500, Aerosil®200, StarLac®90 (Meggle Pharma, Evonik, Wasserburg, Germany). They were donated by Etilfarma. Microcrystalline cellulose (MCC), Vitamin E, Betan F®, Lactic acid, Rose essence, Low density Hyaluronic acid and Magnesium stearate were purchased from Droguería Saporiti S.A.C.I.F.I.A. The LC-MS analysis were performed on an ACQUITY H–Class UPLC instrument equipped with a XEVO TQ-S Micro triple quadrupole mass spectrometer (Waters Corp, Milford, MA, USA) with electrospray ionization (ESI). The particle surface was analyzed using a Scanning electron microscope (SEM) (LEO, 1450 VP, ZEISS, United States). Compression machine, Riva, Piccola D model, with 8 stations, Dual Drum Friability Tester (Electrolab, EF-2, Electrolab, India), pH meter (Orion model 520 A, Orion Research Inc. Boston MA 02129 USA) and Tablet Hardness Tester (DRS Pharmatron, Tablet Tester 8M, DRS, Switzerland) were employed.
The aerial parts of L. divaricata Cav. were collected in February, 2019 in Nogolí, San Luis, Argentina. A voucher specimen was deposited at the Herbarium of the Universidad Nacional de San Luis. The vegetable material was dried in shade at room temperature, then chopped and ground to fine powder in a mechanical blender.
An ethanolic extract was prepared from the aerial part of L. divaricata (500g) following official methods described by FA VII Ed., 2003 [13]. The solid residue (SR) content of fluid extract was determined by evaporation of the organic solvent under reduced pressure, and then dried in an oven (40 °C) to constant weight. (LdE SR= 13.10 g/mL).
An UPLC ACQUITY BEH C18 (1.7 μm, 2.1 mm × 50 mm) column was used for separation at 38 ºC. The mobile phase consisted of an isocratic mixture of 0.1% Formic Acid:Methanol 40:60 as the mobile phase with a flow rate of 0.2 ml/min during 20 min. The injected volume was 20 μl. The calibration curve was constructed by using four standard solutions of NDGA (1.3, 6.5, 13, and 26 ppm) in triplicate. The LdE was prepared at 96 ppm. The samples were dissolved in a mixture of methanol:water (50:50) and filtered through a membrane filter (0.22 µm). The data were acquired using the modes MS2 scan and daughters of m/z 303 [M+H]+ for the chemical characterization and NDGA quantification, respectively. The MassLynx Software V4.2 (TargetLynx™, Waters, Milford, MA, USA) was used for data processing. The amount of NDGA in the LdE was calculated through the calibration curve.
The LdE was mixed with TRICAFOS® 500, AEROSIL® 200 and AEROPERL® 300 in the proportions 1:2 and 1:3 respectively by the impregnation method to obtain the AHI. These dispersions were analyzed by SEM.
The powder blends corresponding F1 and F2 were prepared using LdE + TRICAFOS® 500 (LdET), StarLac® 90, Vitamin E, Betan® F, Microcrystalline cellulose, Lactic acid, Low-density Hyaluronic acid, Rose essence, Magnesium stearate. All of them, except Magnesium stearate, were sieved through a #30 mesh and then mixed for 10 minutes.
Subsequently, Magnesium stearate was sieved using a #40 mesh and added to the previous powder and mixed for 4 minutes.
To determine the density of the mixture of LdET and excipients, the powder was gently poured into a 10 cm3 graduated cylinder to a total volume of 10 cm3. The bulk density (BD) was calculated as the ratio between weight (g) and volume (cm3). To determine the tap density (TD) the cylinder was tapped over 1.0 inch vertical drop, at 1s interval, until no measurable change in volume was noticed. The compressibility of the powder was evaluated using the Hausner Ratio (HR) [14]:
HR= [TD/ BD]
The results are given in table 1.

Table 1: Micromeritic parameters of physical mixtures containing LdET.
The dynamic for mixture of powder was determined by the funnel method as described in the literature [15].
The results are given in Table 1.
The proposed formulas were compressed using a rotary tableting machine, Riva, model Piccola D with 8 stations, with a set of 14.0 mm punches (automatic and continuous operation tableting machine, production 500 tablets per minute, 1.5 kW single-phase motor, maximum compression force 60 kN). Tests of uniformity of weight, hardness, disintegration and friability were conducted in accordance with the methods derived from a compilation of different pharmacopeias. The theoretical weight of each tablet design was 1500 mg and 1200 mg. (Table 2).
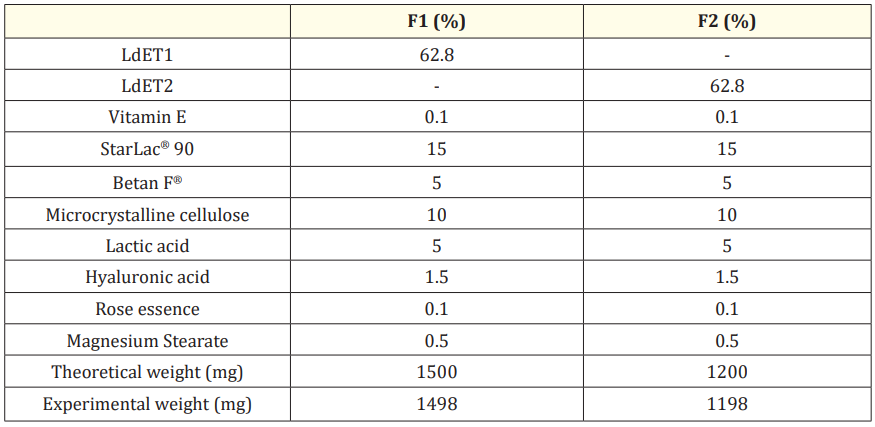
Table 2: Composition of soap herbal formulations containing LdET
The tablets weight variation test was carried out according to USP-43 [16]. Twenty tablets were individually weighed. The results were expressed as the mean value of 20 determinations (Table 3).
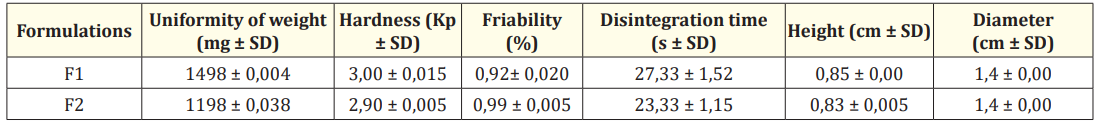
Table 3: Quality and quantity performed on LdET soap herbal tablets.
Ten tablets were selected at random; their surfaces were cleaned to remove adhering dust, weighed and placed in the Friabilator USP. They were then allowed to fall freely 100 times from a height of 6 inches at a speed of 25 rpm for 4 min. The tablets were then dusted and weighed. Any loss in weight due to fracture or abrasion was recorded as a percentage weight loss. The replicate determinations of each formulation were averaged. The test was performed by triplicate (Table 3). The percent friability was calculated as follow:
% Friability= [initial weight- final weight]/ initial weight x 100.
The breaking point and structural integrity of tablets were performed using Pharmatron, Dr. Schleuniger, tablet tester 8M. The evaluation involved conducting 20 determinations to establish the average hardness of the tablets, measured in kilopondio (Kp) (Table 3).
The disintegration test was performed in accordance with USP-43 guidelines. Six tablets were placed individually in each tube of disintegration test apparatus. The water medium was maintained at a temperature of 37 ± 2ºC and the time was noted for the entire tablet to disintegrate completely (Table 3).
Approximately 50 mL of the 1:10 solution of the formulation in distilled water was transferred into a 250 mL graduated cylinder, then the capped cylinder was manually shaken 10 times at a 90º angle. At the end of the agitation, the indicated volume was recorded at the maximum height of the foam formed. After 10 minutes, the foam height was recorded again. The volume of the foam was determined by subtracting the indicated volume from the maximum height of the volume immediately after stirring, and the maintenance of the foam was verified after 10 minutes [17] (Table 4).
The pH of 10% v/v soap herbal tablet solution in distilled water was measured by using pH meter Orion at room temperature [18] (Table 4).
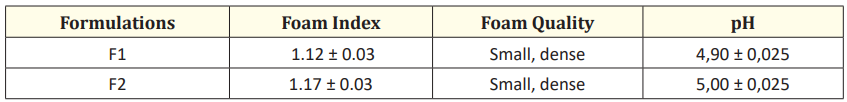
Table 4: Soap herbal tablets control test.
The MIC of LdET against C. albicans is calculated. Young cultures of C. albicans ATCC 10231 less than 18-24 h were used. From a suspension with OD=1.3, dilutions were made to a final concentration of 10-3. CASO Broth® was used as a culture medium to promote enrichment. Miconazole was used as positive controls for C. albicans. Serial dilutions of LdET1 and LdET2 were made (1/10, 1/100, 1/1000, 1/10,000, 1/20,000, 1/40,000, 1/60,000, 1/80,000 and 1/100,000). Each dilution was made in a test tube with a final volume of 10 ml. In addition, a 1/10 dilution of TRICAFOS® 500 was made to identify whether it could be an inhibitor for C. albicans. Once the dilutions were made, 100 µl of the 10-3 dilution of C. albicans was inoculated into each tube of product. A tube of CASO Broth® was added as a growth control. It was incubated for 48 h in an oven at 33ºC. To check microbial viability, tests were made on Tryptone Soy Agar (TSA) medium and incubated at 33 ºC for 48 h.
All results were expressed as mean values ± standard deviation (SD) R software (R version 4.1.0 and RStudio version 1.4.1717) was used to determine the level of significance (p ≤ 0.05) by analysis of variance (ANOVA).
The current study has designed and developed new soap herbal tablets containing L. divaricata, which exhibit activity against candidiasis for vaginal application. (Figure 1).

Figure 1: Soap herbal tablet from L. divaricata.
The AHI was characterized for UHPLC. In the chromatogram the peak at 2.55-2.56 m/z was attributed to NDGA (Figure 2). Considering that NDGA presents toxicity at values greater than 41 µg/ml [19] and in order to determine the security and efficacy of the herbal product obtained, its quantification was necessary. Thus, the content of NDGA in the LdE was performed by UHPLC- MS/MS giving a value of 12.92 ± 1.2% (w/w). The linear correlation curve was r 2 = 0.991879, and the linear equation was y = 1.3713e 6 x + -85118.6.
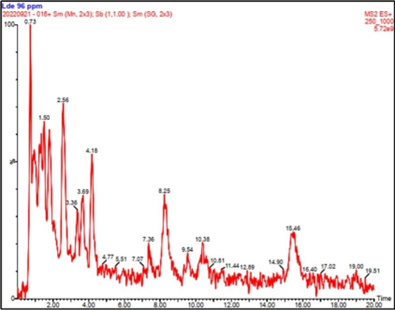
Figure 2: Chemical profile of the ethanolic extract of L. divaricata.
In order to find a carrier that would allow effective loading of the extract, a comparative study between AEROSIL® 200, AEROPERL® 300 and TRI-CAFOS® 500 was carried out. AEROSIL® 200 is a hydrophilic fumed silica with a large specific surface area and AEROPERL®300 is a high purity colloidal silicon dioxide. TRI-CAFOS® 500 is a spray-dried, directly compressible tribasic calcium phosphate with nearly spherical particles. Two relations, carriers: LdE (2:1 and 3:1), were evaluated. The relation 3:1 presented the best rheological parameter. The uniformity of fluid extract distribution was confirmed through a scanning electron microscope (SEM). Figure 3 shows the SEM of AEROSIL® 200 (3a), AEROPERL® 300 (3b) and TRI-CAFOS® 500 (3c). AEROPERL®300 particles are smooth and spherical, TRI-CAFOS® 500 particles appear rough and spherical while AEROSIL® 200 shows irregular particles. The extract-carrier combinations are shown in figure 4. The best distribution of LdE was observed with AEROPERL® 300 (Fig 4b) and TRI-CAFOS® 500 (Fig 4c). TRI-CAFOS® 500 was selected based on the cost/galenic benefit ratio.

Figure 3: Scanning electron microscopy images of AEROSIL® 200 (a), AEROPERL® 300 (b) and TRI-CAFOS ® 500 ©.

Figure 4: Scanning electron microscopy images of L. divaricata extract with AEROSIL® 200 (a), AEROPERL® 300 (b), TRICAFOS ® 500 (c) in a 1-3 ratio.
The qualitative and quantitative composition of the soap herbal tablets is shown in table 2. For the development of this new herbal cosmetic form with potential use in the treatment of vaginal candidiasis, was used as the active ingredient LdET. Starlac® 90, a co-processed lactose excipient, was used to obtain fast-disintegrating tablets. This innovative formulation allowed the incorporation of several pharmacological and pharmacotechnical adjuvants: vitamin E was included for its antioxidant properties, Betan F® as a surfactant and foaming agent, lactic acid as a good pH regulator and low-density hyaluronic acid as humectant. This combination of ingredients was designed to maximize the efficacy of the product and improve its functionality in the complementary treatment of vaginal candidiasis.
The micromeritic properties for physical mixtures are shown in table 1. Both formulations presented optimal fluidity and compressibility conditions with an angle of repose < of 25º and a compressibility percentage < 15%.
Soap herbal tablets of L. divaricata were obtained by direct compression and the experimental results of the quality tests performed are shown in table 3. The weight variation for F1 and F2 was less than 5%, as established by the USP43. Hardness, friability and disintegration time tests give an idea of the mechanical strength of the tablets, related to deterioration during transportation, packaging and handling. F1 and F2 presented values for hardness, friability and disintegration time, in concordance with the official parameters. The optimal values of disintegration time, less than 30 seconds, were attributed to the properties of Starlac® 90.
In relation to the characteristics of the tablet as soap, foam is not a critical property in the case of vaginal soaps. However, its presence may indicate that the soap is working correctly in terms of creating a solution that effectively distributes the active product to the surfaces being cleaned. The results of the foam tests are shown in table 4. The foam was of the closed or dense type with an index of 1.12 and 1.17 for F1 and F2, respectively. Dense foam provides better coverage of the soap on the skin and allows for uniform and controlled application in specific areas, minimizing soap waste. In addition, closed foam tends to reduce irritation in delicate areas such as the vaginal area.
For intimate hygiene soaps, it is crucial to maintain the pH balance to ensure the health of the vaginal area. The ideal pH for a product intended for vaginal use should be between 4.5 and 5.5. The pH values obtained for formulations F1 and F2 were 4.9 and 5.0, respectively (Table 4). Lactic acid, present in both formulations as a pharmaceutical adjunct, not only regulates the pH and protects the vaginal microbiota but also prevents the growth of microorganisms that can cause unpleasant odors.
The antimicrobial assay demonstrated that a 1/10 dilution of LdET1 was able to inhibit the growth of C. albicans. (Figure 5). The MIC, defined as the lowest concentration of extract capable of inhibiting the growth of C. albicans, was 25 mg/ml. TRI-CAFOS® 500 did not present antimicrobial activity. The results are shown in table 5.

Figure 5: Antimicrobial activity LdET1 against C. albicans by TSA agar dilution method (Dilutions G: 1/10, H:1/100, I: 1/10000, J: 1/20000, K: 1/60000, L: 1/80000).
In conclusion, F1 achieved optimal quality indicators and showed activity against C. albicans. This formulation could be considered as a new cosmetic form free of parabens, friendly to the environment, suitable for vaginal hygiene contributing to treatments for candidiasis.
This work was supported by UNSL (PROICO 02-1423), CONICET and CICITCA UNSJ.
The authors declare no conflict of interest.
Copyright: © 2024 Raksha Upreti.,et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.