Ezenwa Chika Maureen* and EZENWA CM
IMO State University, Owerri, IMO State Nigeria
*Corresponding Author: Ezenwa Chika Maureen, IMO State University, Owerri, IMO State Nigeria.
Received: July 22, 2024; Published: September 05, 2024
Citation: Ezenwa Chika Maureen and EZENWA CM. “Analytical Studies on Microbial Diversity of (AGIDI) a Traditional Delicious Consumed Fermented Food. By Dr Ezenwa C.M Imo State University Owerri. Faculty of Biological Science". Acta Scientific Nutritional Health 8.10 (2024):02-08.
This study investigates the microbial diversity of agidi, a traditional fermented cereal product widely consumed in West Africa. The analysis focused on the bacterial and fungal loads of agidi samples, as well as the identification and characterization of bacterial and fungal isolates present in the samples. The results revealed a diverse microbial community comprising bacteria such as Klebsiella spp, Lactobacillus spp, Pseudomonas spp, Bacillus spp, and Staphylococcus aureus, alongside fungal species including Saccharomyces spp and Candida spp. Total heterotrophic bacteria counts ranged from 3.2 x 10^4 to 6.0 x 10^6 CFU/g, while total coliform counts ranged from 1.6 x 10^4 to 5.0 x 10^6 CFU/g. Similarly, total staphylococcal counts ranged from 6.0 x 10^5 to 4.2 x 10^6 CFU/g, and total fungal counts ranged from 4.0 x 10^3 to 6.5 x 10^6 CFU/g. The identification and characterization of bacterial isolates based on colonial and morphological characteristics, as well as biochemical and physiological tests, provided insights into the diversity and composition of the microbial community in agidi samples. This study contributes to the understanding of microbial dynamics in agidi production, which is essential for optimizing fermentation processes and ensuring product quality and safety.
Keywords: Microbial Diversity; Traditional; Delicious; Fermented Food; Biological
Cereals belong to the monocot families, "Poaceae or Gramineae" and are cultivated widely to obtain the edible components of their fruit seeds. Botanically, these fruits are called "caryopsis” and are composed of endosperm, germ and bran. Cereal grains are grown in greater quantities and provide more food energy worldwide than any other type of crops. They are therefore called staple crops [1]. In tropical Africa, cereal grains are milled and used to produce thick porridges, which are known by various names in different parts of the continents. In West Africa particularly in Nigeria, Ghana, Burkina Faso, one of such thick porridges is called fura, a semi solid dumping cereal meal, [2]. Cereal grains are the major sources of proteins for people of Nigeria. Those receiving less than 20% of the calories and protein intake from cereals mainly consist of people in Southern Nigeria where starchy roots and tubers are staple foods [3].
Agidi is a gel-like traditional fermented starchy food item produced from (Zea mays). Although, millet and sorghum can also serve as raw materials, its colour depends on the cereal used. It is cream to glassy white from maize, light brown from sorghum and grey to greenish from millet. It is known by different localities such as "eko"(Yoruba)"komu"(Hausa)"Auii'(ho) [4] Agidi is prepared by wet sieving with the aid of clean cloth. The mixture is allowed to settle and the ogi shurry supematant decanted, boiled and cooked with occasional stirring to get agidi meal. Agidi ean be prepared as "jolloff agidi" which can be consumed at any time without the requirement of stew [5].
The major cereals in Nigeria include Maize, Sorghum, Millet and Rice. Cereals are utilized in various ways in Nigeria. Maize is utilized in different ways such as ogi. Ogi is a poridge prepared them fermentation of maize in Nigeria. It is a staple food that can be served as a' weaning food for infants or breakfast for adults., Ogi" can also be prepared from millet and sorghum. Pito which is produced trom maize is a traditional alcoholic drink in Nigeria. Popcorn (guguru), egbo, agidi, moi-moi, and tuwo are also produced from maize, Sorghum is utilized in different ways which include production of burukutu - a local alcoholic beverage in Nigeria. The process involves malting, mashing, boiling, fermentation and maturation. It can also be produced using a combination of maize and sorghum. Kunun zaki is also produced from sorghum which can be combined with millet or maize depending on individual preference. Pito and Ndaleyi are as well made from sorghum
[6] Millet is one of the major cereals utilized in Nigeria to produce kunun zaki, fura and the process involves decortication/dehulling, washing, drying, milling, fermenting, moulding, steaming, and drying. Masa, pito, ogi are all produced from millet. Rice is one of the major cereals in Nigeria which is used to make tuwo and can be consumed with beans soup and masa. It is used to produce kunun by mixing it with millet. The tropical Africa has many cereal crops [7] with diverse utilization most of which are not properly documented.
Fermentation of cereal based foods is a common practice in Africa for food preservation. It is a technology that is simple; home based and has fed millions of people. Currently, a variety of fermented foods are produced from cereals at house hold and semi industrial scale. These foods are used as weaning food for infants and children [8] Lei and Jacobsen 2004) and also for adults.
This study is aimed at studying the microbial diversity of Agidi a fermented cereal product Objectives of study includes the following. To determine the microbial load of ready to eat agidi sample purchased from different locations in Owerri, to isolate the microbial from ready to eat agidi sample purchased from different locations in owerri and to identify the isolated microbes using a combination of morphological and biochemical characteristics.
Agidi, a tradiional West African fermented cereal product, holds significant cultural and dietary importance in regions like Nigeria [9]. Typically made from maize, millet, or sorghum flour, agidi undergoes a fermentation process that involves soaking the grains, grinding them into a paste, and allowing spontaneous fermentation to occur (Adebayo [10]. This process results in a gelatinous porridge-like consistency, making agidi a staple food in many West African households.
Microbial diversity is crucial in the fermentation of agidi and similar foods, impacting various aspects of their quality and safety [11]. Lactic acid bacteria (LAB) dominate the fermentation process, contributing to acidification, which preserves the product and inhibits the growth of harmful bacteria [12]. Yeasts and molds also play essential roles, contributing to flavor development through the production of aromatic compounds and ethanol, and enhancing digestibility through carbohydrate breakdown [13]. Understanding microbial diversity is vital for optimizing production processes and ensuring product consistency and safety [14]. However, microbial diversity can also present challenges, as undesirable microorganisms may lead to spoilage or foodborne illnesses [15].
Agidi production traditionally involves a series of steps that rely on indigenous microorganisms and natural fermentation processes. The process begins with soaking maize, millet, or sorghum grains in water for a period to soften them. The softened grains are then ground into a paste or flour, which is mixed with water to form a thick slurry. This slurry is left to ferment spontaneously at ambient temperatures, typically for a period ranging from 24 to 72 hours, depending on local practices and environmental conditions. During fermentation, microbial populations naturally present in the environment, such as lactic acid bacteria, yeasts, and molds, proliferate and metabolize carbohydrates present in the grains, leading to the acidification of the mixture and the development of characteristic flavors and textures associated with agidi [16].
The fermentation of AGIDI is driven by a diverse community of microorganisms, including bacteria, yeasts, and molds, which play essential roles in the transformation of raw materials into the final product. Lactic acid bacteria (LAB), such as Lactobacillus species, are predominant during fermentation and are responsible for acidifying the mixture through the production of lactic acid. This acidification helps to lower the pH of the environment, creating conditions unfavorable for the growth of pathogenic bacteria and contributing to the preservation of the product. Yeasts, including species like Saccharomyces cerevisiae, contribute to flavor development by producing aromatic compounds and ethanol through the fermentation of sugars present in the grains. Molds may also be present during fermentation and can contribute enzymes that break down
Six samples each of Agidi was bought from different vendors and they were used for the analysis
Food samples were aseptically collected using a polythene bag and they were transported to the microbiology lab in Imo State University within one hour after collection.
The materials that were used in this research work include polythene bags, measuring cylinder, conical flasks, petri dishes, sterile glass slides, forceps, wire loop, filter paper, disposable hand gloves, pipette, test tubes, test tube rack, media, autoclave, incubator, weighing balance, Bunsen burner, distilled water, 75%ethanol, crystal violet, iodine and light microscope, Methyl red, Kovac Reagent, Crystal violet, Safranine.
The culture media that were used in this research work include: Nutrient agar, Mannitol salt agar, salmonella-shigella agar, Maconkey agar, and Sabouraud dextrose agar.
The samples were labeled with masking tape and pen on reaching the microbiological lab. The various samples were represented with alphabets to enable easy identification and used to represent the various samples of agidi samples used
All glass wares to be used were sterilized after being washed with detergent using autoclave. The Nutrient agar, Mannitol agar, MRS agar, and MacConkey agar were also sterilized by Autoclaving at 121°C, 15PSI, while salmonella-shigella agar was boiled according to the manufacturer's instruction. Wire loops were sterilized by flaming to red hot using Bunsen burner and all laboratory benches shall be cleaned before and after work with 75% alcohol. Bunsen burner shall be lit during the work to keep the environment sterile.
10g each of the agidi samples were dispensed in 90ml of sterile distilled water and used for 10 fold serial dilution after which 0.1ml of the 104dilution was inoculated into a sterile petri dish containing 20ml Of freshly prepared molten Nutrient agar using spread plate method of inoculation by spreading the inoculums using a sterile bent glass rod after which the media were incubated for 24hrs at 37℃ for aerobic bacterial isolation media. After the incubation period, the plates were observed and colonies counted and plates which had colonies ranging from 30-300 colonies were used, and the discrete coloies from the cultured plates were sub-cultured into a freshly prepared Nutrient agar plate to get a pure culture for bacteria isolates. The sub-cultured plates were incubated for 24hrs for aerobic bacteria, and examined for pure culture. The pure culture growth was used for gram staining, motility test and biochemical characterization of the organisms like Oxidase tests, Citrate utilization test, Indole test, Methyl-red test, Voges proskaeur test, Coagulase test, Sugar fermentation and Catalase test. A stock culture was prepared using a bijou bottle: this stock culture was used in storing the organisms for further biochemical characterization., 10g each of the agidi samples were dispensed in 90ml of sterile distilled water and used for 10 fold serial dilution after which 0.1ml of the 10-dilution was inoculated into a sterile petri dish containing 20ml Of freshly prepared molten MacConkey agar using spread plate method of inoculation by spreading the inoculums using a sterile bent glass rod after which the media were incubated for 24hrs at 37℃ for aerobic bacterial isolation media. After the incubation period, the plates were observed and colonies counted and plates which had colonies ranging from 30-300colonies were used, and the discrete colonies from the cultured plates were sub-cultured into a freshly prepared Nutrient agar plate to get a pure culture for bacteria isolates. The sub-cultured plates were incubated for 24hrs for aerpbic bacteria and examined for pure culture. The pure culture growth was used for gram staining, motility test and biochemical characterization of the organisms like Oxidase tests, Citrate utilization test, Indole test, Methyl-red test, Voges proskaeur test, Coagulase test, Sugar fermentation and Catalase test. A stock culture was prepared using a bijou bottle: this stock culture was used in storing the organisms for further biochemical characterization. 10g each of the agidi samples were dispensed in 90ml of sterile distilled water and used for 10 fold serial dilution after which 0.1ml of the 10-dilution was inoculated into a sterile petri dish containing 20ml Of freshly prepared molten Sabouraud dextrose agar using spread plate method of inoculation by spreading the inoculums using a sterile bent glass rod after which the media were incubated 28C for 72hours After the incubation period, the plates were observed and colonies counted and plates which had colonies ranging from 30-300 colonies were used, and the discrete colonies from the cultured plates were sub-cultured into a freshly prepared sabouraud dextrose agar plate to get a pure culture for fungal isolates. The bacterial isolates were identified using colonial, cellular characteristics, Gram Staining, Motility test and biochemical properties. Biochemical test carried out include; Urease test, Citrate Utilization test, Inole test, Methyl-Red test, Coagulase test, Sugar test and Catalase. Colonial and cellular characteristics were used in the identification of microbial isolates and they include; Colony's shape, colour, consistency, surface appearances and size; Size of the colony (diameter in mm). Shape or form of the colony oil immersion objective (X100). Gram positive cells showed purple while gram negative cells showed red colour.
The method described by Cheesbrough (2006) was adopted. It is used to differentiate between motile and non-motile organisms due to the presence of locomotory structures like flagella. This test was carried out using the stab method. Test tubes of semi-solid motility medium were inoculated by stabbing a sterile straight wire loop charged with inoculums from the isolated pure culture vertically into the media and it was incubated at 37℃ for 24hours. Non-motile bacteria produced growths that were un-diffused from the line of stab while motile bacteria produced diffused growth away from the line of stab into the medium and rendered it opaque.
Some of the biochemical tests to be used in identification bacteria will include
This is a test used to differentiate catalase producing bacteria like Staphylococci from non catalase producing bacteria such as Streptococci. The catalase produced acts as a catalyst in the breakdown of hydrogen peroxide to oxygen and water, the method as described as by [18] was adopted. A drop of 3%adopted. A drop of distilled water was placed on each end of the microscope slide. A colony of test organism was emulsified in each of the drops of distilled water that was placed on the ends of the microscopic slide, to make thick suspensions. A 100cfu/l of plasma was added to one of the suspension and mixed gently. No plasma was added to the same suspension serving as control. Clumping of the mixture within 10 seconds will indicate positive coagulase test, while absence of clumps within 10seconds indicates a negative result
The method of Cheesbrough (2006). was adopted. Testing for indole production is important in the identification of enterobacteria. The test organism is cultured in a medium which contains tryptophan. Indole production is detected by Kovac's eagent which, contain 4-p-dimethylaminobenzaldehyde; it reacts with the indole o produce a red coloured compound. The test organisms were inoculated in a bijou ottle containing 3ml of sterile tryptone water, which was incubated at 37℃ for 8hrs, after incubation, 0.5ml of kovac's reagent was added, the tubes were gently haken, and the appearance of a red surface layer within 10mins indicates a ositive indole test.
e test is used to assist in the identification of Pseudomonas, Neisseria, Vibrio, ucella, Pasturella species, all of which produce the enzyme cytochrome oxidase (Cheesbrough, 2006), The method as described by Cheesbrough (2006) was adopted. A piece of filter paper was soaked with a few drops of oxidase reagent (tetra-ethyl-p-phenylendeamine dihydrochloride). A colony of the test organism was picked with a sterile glass rod and smeared on the filter paper. A blue purple colour develops within a few seconds if the organism is an oxidase producer as a result of the oxidation of the phenylendeamine, while the absence of a blue purple colour indicates a negative result.
This test is used to determine which fermentation pathway is used to ructos glucose [19] It is used to differentiate bacteria that are capable of fermenting glucose with the production of enough acid to lower the Ph of the medium to 4-4.5 and that ferment glucose without much acid production. Methyl red contains glucose and peptone. The method as described by [20] was adopted. The bacteria isolates were inoculated into 2mls of glucose phosphate (peptone water) and was incubated at 37°C for 48hours. After the period of incubation, 4 drops of methyl red indicator was added to the tube. The solution was ructoseed and observed immediately for colour change. The appearance of a red colour indicates a positive result while the appearance of a yellow colour indicates a negative result [20]. For Voges proskauer test, the method described by [20] was adopted, the bacteria isolates were added to 2ml of glucose phosphate (peptone water) and it was incubated al 37℃ for 4Shrs, after incubation, 40% KOH and 3ml of 5% alcoholic alpha naphthol were added, the appearance of apink colour after 2-5 minutes indicates a positive result.
This test was employed to check for the ability of an organism to ferment sugar. The agar used in this test is called Triple Sugar Iron (TSI). This test engines the shility of the organism to produce gas, Hydrogen sulphide, to ferment Glucose, maltose and ructose to also ascertain if its Slant and Base are acidic or basic, The agar was sterilized at 121°C at 15mins. The test organism was inoculated at a slanted test tube. A colour change from purple to yellow indicates the utilization of several sugars. A black duct at the slanted area indicates the presences of H2S. Also a gaseous bubble at the bottom or slant of the test tube indicates the presence of gases while displacement in the durham's tube indicates gas production [20].
The fungal isolates were identified using cultural and morphological features such as colony growth pattern, conidial morphology and pigmentation for macroscopic examination and wet mount technique using lactophenol cotton blue for microscopic examination of the fungal isolates
The result shows the bacterial load of the various agidi samples. The various samples were represented with alphabets A, B, C, D, E, and F. The total heterotrophic bacteria count ranged from 3.2 x 104 to 6.0 x 10°, the total coliform count for the Agidi samples ranged from 1.6 x 104 to 5.0 x 106. The total staphylococcus count for the samples ranged from 6.0 x 105 to 4.2 x 106. Total fungi count for the samples ranged from 4.0 x 103 to 6.5 x 106. The reséarch also reveals that the bacterial isolates were Klebsella spp, Lactobacillus spp, Pseudomonas spp, Bacillus spp, and Staphylococcus aureus.
Percentage occurrences of isolates showed that Bacillus spp had a percentage occurrence of 29, Klebsiella spp had a percentage occurrence of 20, Lactobacillus spp had a percentage occurrence of 13, pseudomonas spp has a percentage occurrence of 21 and Staphylococcus aureus had a percentage occurrence of 17.
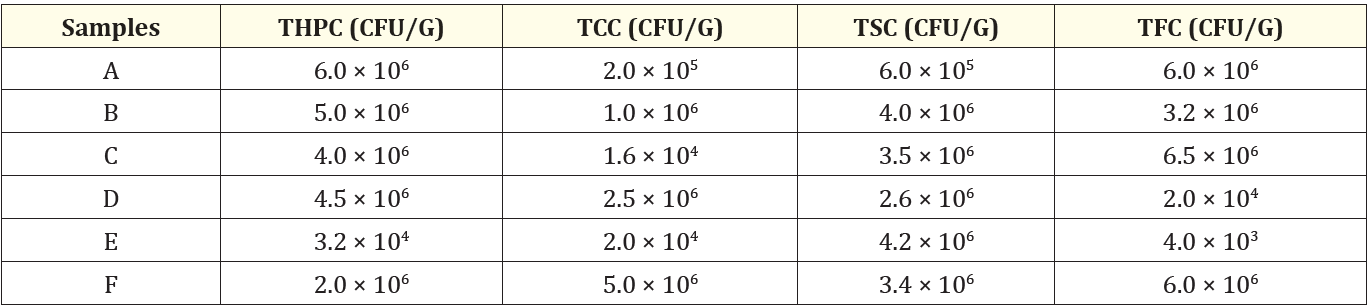
Table 1: Bacteriological loads of samples.
THPC: Total Heterotrophic Plate Count
TCC: Total Coliform Count
TLC: Total Staphylococcal Count
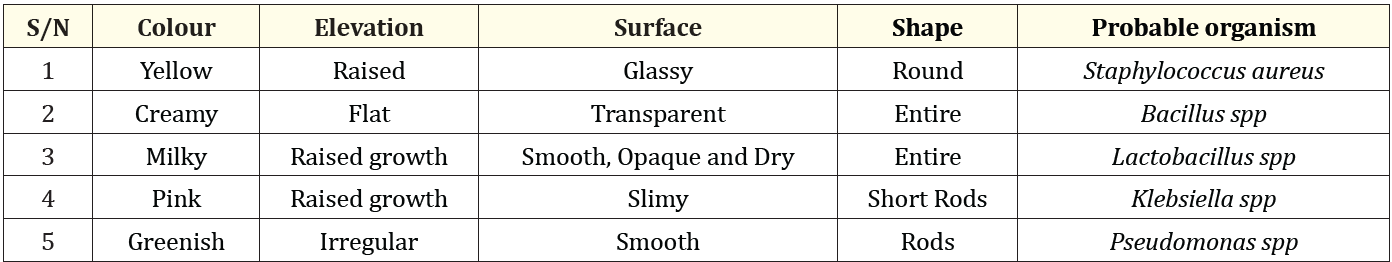
Table 2: Colonial and bacteriological characteristics of bacterial isolates.
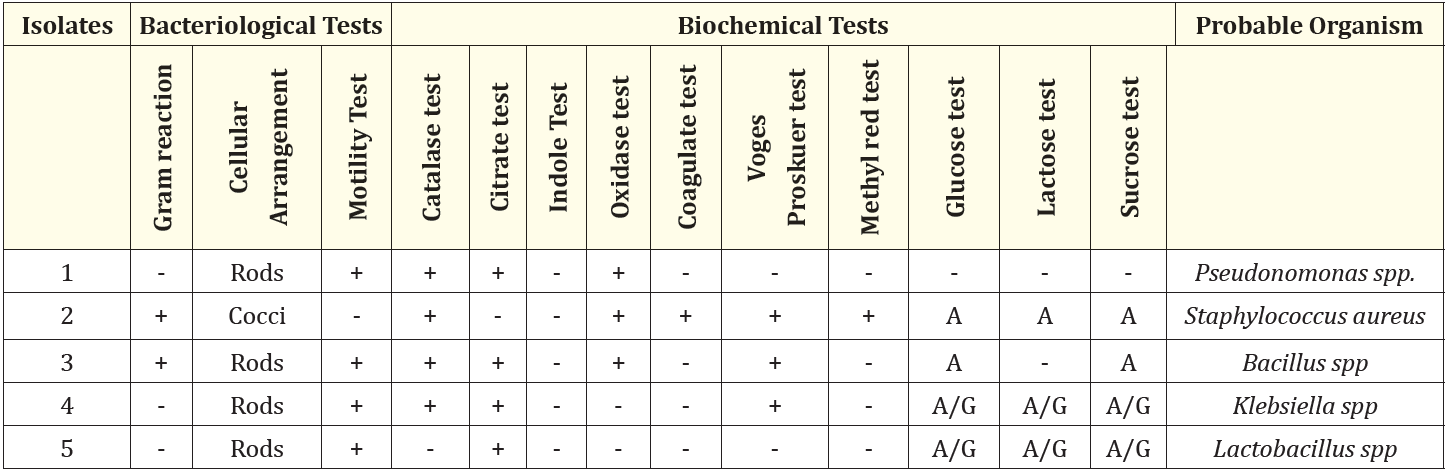
Table 3: Physiological properties of bacterial isolates.
- = Negative
+ = Positive
A = Acid
A/G= Acid and Gas Production
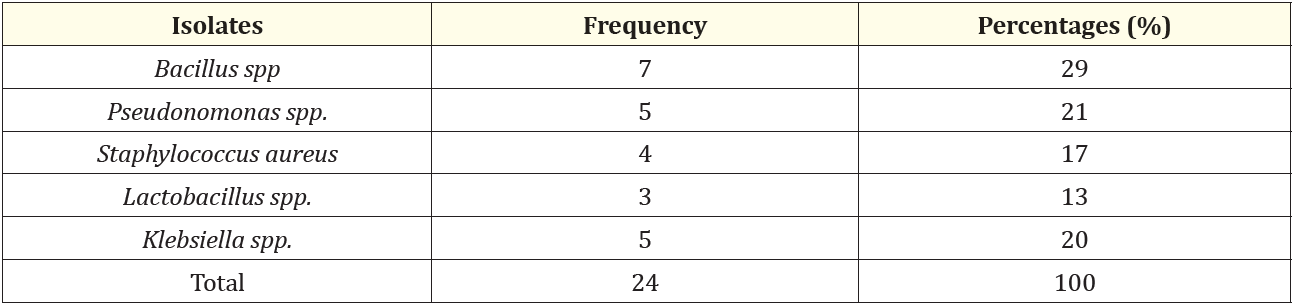
Table 4: Frequency of occurrence of bacterial isolates.

Table 5: Colonial morphology and microscopic morphology of fungi species.
The isolation of lactic acid bacteria are due to the fact that they are responsible for the fermentation of most legumes and cereals. Their presence also confirms that they grow in close association with the food substrate (Steinkraus, [21]. These organisms are obligate fermenters, flavorful organisms not harmful to the consumers but produce enzymes that hydrolyze food complexes into simple non- toxic products with desirable textures and aroma that makes them palatable [22,23]While the presence of other organisms could be through various sources especially when strict hygienic practices are not adhered to, which could be attributed to the raw materials used, processing environment, human involvement, milling machine employed, muslin clothes used in filterng, source of water and utensils used [24].
Staphylococcus species isolated is understandable since it is a normal flora of human body which could be transferred into the product during processing. Staphylococcus aureus has been reported to remain the most prominent etiology of pyogenic infections and that staphylococcal infection leads to a worsening of some already existing superficial infections (Adegoke and komolafe, 2009)
The isolation of the various bacteria from the "pap" samples confirmed that it could serve as a vehicle for the transmission of potentially pathogenic microorganisms. Since a total of five bacteria were isolated during experiment, caution should therefore be taken by both the producers and the consumers concerning this dependable food product called “pap”, in order to ensure that health benefits are conferred on the consumers while the shelf life of "pap" is elongated by the absence of those microorganisms that bring about decomposition. It is therefore recommended that the following strategies should be mapped out and embarked upon in order to reduce to a tolerable level or totally eliminate bacterial contamination on “pap”. Some of these strategies have been approved as regulations by the Department of Health Education and Welfare, Public Health Services and Food and Drug Administration (FDA) and local agencies over current trends in the manufacturing, processing, packaging or holding of human food generally referred to as GMPs (Good Manufacturing Practices)
Finally, the government should also introduce the use of probiotics to the local producers of “pap” and sell to them at a very subsidized rate. The probiotics are meant to mimic the normal microbial flora in humans like the ones found on breast milk that offer protection against diseases. The most frequently used ones for now are Lactobacillus species and Bifidobacterium species. More research should be done to introduce more of these probiotics.
Copyright: © 2024 Ezenwa Chika Maureen and EZENWA CM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.