Natasha R Marak* and Mothanro M Odyuo
Department of Food Science and Nutrition, College of Community Science, Central Agricultural University (Imphal), Sangsanggre, Meghalaya, India
*Corresponding Author: Natasha R Marak, Department of Food Science and Nutrition, College of Community Science, Central Agricultural University (Imphal), Sangsanggre, Meghalaya, India.
Received: August 12, 2024; Published: August 29, 2024
Citation: Natasha R Marak and Mothanro M Odyuo. “Non-Celiac Gluten Sensitivity: Unraveling the Complexities". Acta Scientific Nutritional Health 8.9 (2024): 100-104.
Gluten sensitivity spans a spectrum of reactions, ranging from mild discomfort to severe symptoms, triggered by the consumption of grains containing gluten such as wheat, barley, and rye. Unlike celiac disease, diagnosing gluten sensitivity is difficult due to the absence of specific markers. Symptoms can affect the gastrointestinal system, neurological functions, and other body systems, including abdominal discomfort, bloating, fatigue, headaches, and cognitive issues.
Studies have shown that symptoms are caused by a complex interaction of genetic predisposition, intestinal permeability, and immune response. Advances in our understanding of gluten sensitivity highlight the importance of ongoing research to improve accurate diagnosis, personalized dietary management, patient care, and quality of life.
Keywords: Gluten; Gluten Sensitivity; Wheat Allergy
The rise in global adoption of the Mediterranean diet, featuring a variety of gluten-rich foods like bread, pasta, and pizza, has led to a concerning surge in gluten-related health issues [1].
Non-celiac gluten sensitivity (NCGS) is a condition characterized by both intestinal and extra-intestinal symptoms triggered by the consumption of gluten-containing foods in individuals without celiac disease (CD) or wheat allergy (WA) [2]. Leccioli., et al. defined NCGS as a complex disorder with potentially temporary and avoidable onset, linked to an imbalanced diet [17]. Due to the absence of reliable biomarkers, the exact prevalence of NCGS is uncertain, though it is believed to be more widespread than celiac disease [3].
Although non-celiac gluten sensitivity (NCGS) is triggered by consuming cereals containing gluten, the specific dietary component responsible has not been pinpointed yet, potentially including proteins other than gluten itself, such as amylase-trypsin inhibitors (ATIs) [4]. There is emerging evidence suggesting the involvement of fermentable oligo-, di-, and mono-saccharides and polyols (FODMAPs) in inducing intestinal symptoms resembling NCGS, like bloating and diarrhea. However, NCGS and FODMAP intolerance may overlap since gluten-rich foods, especially wheat, also contain high levels of FODMAPs. Unlike celiac disease (CD), no genetic predisposition has been identified in NCGS patients, and the mechanisms of the disease remain unclear. Experimental findings indicate a potential role for an abnormal innate response triggered by wheat, as well as changes in small intestinal permeability ("leaky gut"), leading to excessive absorption of gluten-derived peptides [2].
The exact prevalence of non-celiac gluten sensitivity (NCGS) remains unclear. In the US and UK, over 10% of adults initiate a gluten-free diet (GFD) for various reasons and durations, but many cases are self-diagnosed without medical confirmation [10]. Indirect evidence suggests that "true" NCGS may be slightly more common than celiac disease (CD) [11], which affects approximately 1% of the general population [12]. NCGS is predominantly observed in adults, especially females aged 30 to 50 years; however, there have been reported cases in pediatric populations as well [1,13].
Researchers are currently delving into the pathogenesis of non-celiac gluten sensitivity (NCGS), a disorder that has only recently garnered systematic research attention. Our understanding of NCGS pathogenesis is akin to what was known about celiac disease over two decades ago. While gluten is the established trigger for celiac disease, it was initially assumed to be the same for NCGS. However, besides gluten, wheat, barley, rye, and their derivatives contain other components that can provoke symptoms, including amylase-trypsin inhibitors (ATIs) and FODMAPs [29].
FODMAPs may cause mild wheat intolerance, primarily limited to intestinal symptoms, but they can be excluded from further discussion concerning NCGS. Patients with NCGS experience symptom relief upon eliminating gluten-containing grains, even though they may still ingest FODMAPs from other sources [29].
The clinical signs of non-celiac gluten sensitivity emerge following the consumption of gluten-containing grains. These symptoms alleviate or vanish upon removing these grains from the diet and return upon reintroducing gluten, typically within a few hours or days. Gastrointestinal symptoms include abdominal discomfort, bloating, and irregular bowel movements (such as diarrhea, constipation, or both), while additional symptoms may include cognitive difficulties, headaches, joint and muscle pain, fatigue, depression, numbness in the limbs, skin conditions like eczema or rashes, and anemia [15,16].
Several laboratory studies have confirmed the harmful effects of gliadin, the main antigen in gluten, through cytotoxicity. Gliadin proteins exhibit various activities such as agglutination, reducing Factin content, inhibiting cell growth, triggering apoptosis, disturbing redox balance, inducing cytoskeleton rearrangements via zonulin, and causing loss of tight junctions in the intestinal lining [18-20]. The immune response to gliadin differs among individuals. However, patients with non-celiac gluten sensitivity (NCGS) typically exhibit normal levels of intestinal permeability and expression of tight junction proteins, except for an increased expression of claudin-4 [21].
Interestingly, when compared to patients with celiac disease or control groups (patients with dyspepsia), individuals with non-celiac gluten sensitivity (NCGS) exhibit elevated levels of toll-like receptor-2 (TLR2) and TLR4 in intestinal tissues. They also have increased counts of α and β intraepithelial lymphocytes and decreased numbers of T-regulatory cells, while markers of adaptive immunity show no significant differences [21,22]. These observations suggest a potential role of the innate immune system in the development of NCGS.
Amylase-TRIPSIN Inhibitors (ATIs) are albumin proteins found in wheat, constituting up to 4% of the total grain proteins [23]. They exhibit high resistance to intestinal proteases and can trigger the release of pro-inflammatory cytokines from monocytes, macrophages, and dendritic cells by activating toll-like receptor-4 in both celiac diseases (CD) and non-celiac gluten sensitivity (NCGS) patients [23,25]. ATIs have the potential to stimulate innate immune cells and cause intestinal inflammation [24]. Studies, such as those conducted by Junker., et al. on mice deficient in toll-like receptor-4 (TLR4) or TLR signaling, have confirmed the activation of the immunological system by ATIs [25]. These studies demonstrated that mice models lacking TLR4 or TLR signaling were shielded from both intestinal and systemic immune responses when exposed to oral ATIs [23]. Additionally, scientists have found that ATIs stimulate monocytes, macrophages, and dendritic cells in vitro to produce various inflammatory mediators such as IL-8, IL-12, TNF, MCP-1, and RANTES [26].
FODMAPs are carbohydrates with fewer than 10 carbon atoms per molecule [27]. Recent scientific interest has focused on the potential role of FODMAPs in the development of gastrointestinal disorders [28]. Researchers at Monash University in Australia conducted a comprehensive analysis of a group of carbohydrates that, despite having different structures, produce similar effects after consumption. The most common types of FODMAPs include fructooligosaccharides (FOS), galactooligosaccharides (GOS), lactose, fructose, and polyols such as sorbitol and mannitol.
A gluten-free diet (GFD) is the primary treatment for non-celiac gluten sensitivity (NCGS), although it's not yet clear if lifelong adherence is necessary as it is for celiac disease (CD). Research by Carroccio., et al. found that 74% of NCGS patients continued to avoid wheat even eight years after diagnosis, as wheat consumption could still trigger symptoms [30]. While a GFD can alleviate NCGS symptoms, there are challenges with its implementation. It often leads to increased intake of macronutrients like saturated fats, lipids, and sugar, along with reduced intake of micronutrients such as iron, folate, and zinc [31]. Studies have shown that individuals with self-reported NCGS on a GFD consume more saturated fat and less fiber and micronutrients compared to those on a regular diet [32,33]. Additionally, gluten-free products tend to be more expensive, adding to the economic burden [34]. Therefore, clinicians need to carefully evaluate the role of gluten in NCGS development and assess individual tolerance levels to determine if strict adherence to a GFD is necessary [35]. Some experts suggest implementing a gluten rechallenge after 1-2 years on a GFD to determine the patient's tolerance level [42].
Although a strict gluten-free diet (GFD) typically reduces symptom severity in non-celiac gluten sensitivity (NCGS) patients, some individuals continue to experience symptoms despite long-term adherence to the diet [36]. Implementing a low-FODMAP diet has shown promise in reducing symptom severity in NCGS patients [40], but its adoption requires careful consideration due to potential drawbacks. Low-FODMAP diets have been linked to lower intake of natural antioxidants and micronutrients and may affect the balance of beneficial bacteria in the colon [41]. While FODMAPs have been associated with positive effects on lipid metabolism, calcium absorption, and protection against colorectal cancer, supplementation with prebiotics and vitamins is recommended for patients following a low-FODMAP diet [37]. Additionally, close monitoring by a trained dietitian is advised to assess nutritional intake and ensure proper management. Studies have indicated that nutritional intake does not significantly differ between CD patients following a GFD alone and those following both a GFD and low-FODMAP diet when supervised by a trained dietitian [38].
It is advisable to conduct a follow-up assessment after 4 to 6 weeks of initiating a low-FODMAP diet to evaluate the patient's response and determine whether the reintroduction of high-FODMAP foods is appropriate [39]. Generally, the adoption of both a gluten-free diet (GFD) and a low-FODMAP diet in non-celiac gluten sensitivity (NCGS) patients should be considered if there is a noticeable improvement in clinical symptoms. However, it is crucial to seek medical and dietitian guidance to mitigate the risk of nutritional deficiencies resulting from dietary restrictions.
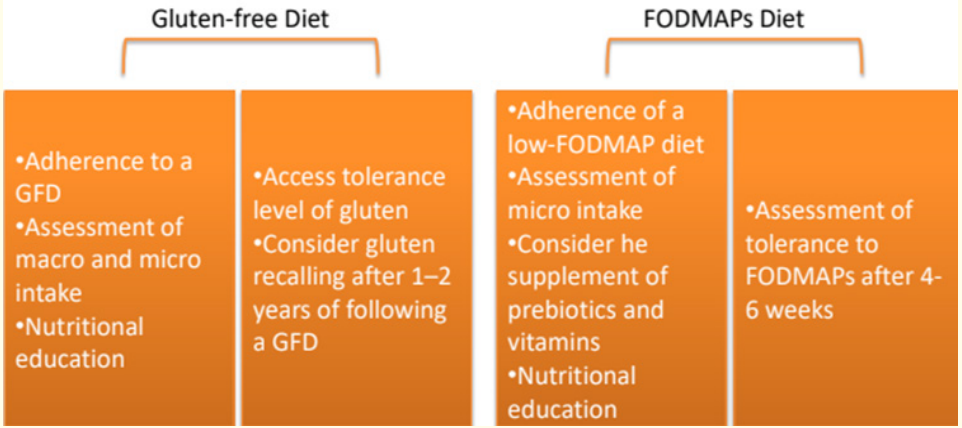
Figure 1: Dietary Management of NCGS.
In summary, our understanding of non-celiac gluten sensitivity (NCGS) is still in its early stages, presenting various challenges for clinicians and researchers in identifying cases. This review provides updated insights into NCGS prevalence, pathogenesis, dietary management, and diagnostic biomarkers. However, the lack of sensitive and reliable biomarkers for diagnosing NCGS and the absence of a standardized diagnostic approach in clinical practice contribute to the uncertainty surrounding its true prevalence. Current evidence on prevalence largely relies on survey studies. Similarly, while there are indications of involvement from both the innate and adaptive immune systems in the pathogenesis of NCGS, further research is needed to fully elucidate its mechanisms.
Copyright: © 2024 Natasha R Marak and Mothanro M Odyuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.