Jia Qiu1, Zhenhuan Liu2 and Alan Wang3
1Jia Qiu, Meixian District Traditional Chinese Medicine Hospital, Meizhou, Guangdong Province, China
2Nanhai Maternity and Children Hospital Affiliated to Guangzhou University of Chinese Medicine, China
3Auckland Bioengineering Institute, Medical Imaging Research Center, Faculty of Medical and Health Sciences, Centre for Co-Created Ageing Research, Centre for Brain Research, The University of Auckland, New Zealand
*Corresponding Author: Alan Wang, Auckland Bioengineering Institute, Medical Imaging Research Center, Faculty of Medical and Health Sciences, Centre for Co-Created Ageing Research, Centre for Brain Research, The University of Auckland, New Zealand.
Received: July 12, 2024; Published: September 28, 2024
Citation: Alan Wang., et al. “Efficacy of Liu's Scalp Acupuncture Therapy in the Treatment of Cerebral Palsy: A Case Report”. Acta Scientific Neurology 7.10 (2024): 17-20.
Cerebral palsy (CP) is a non-progressive brain disorder caused by immature brain development or injury. It results in motor impairment, often accompanied by sensory, perceptual, cognitive, communication, and behavioral disorders. This case report describes the treatment of a 2-year-old male patient diagnosed with cerebral palsy using Liu's scalp acupuncture therapy, a method developed by Professor Liu Zhenhuan. The patient, who had a history of birth asphyxia and developmental delays, showed significant improvement in motor functions after undergoing Liu's scalp acupuncture therapy for five treatment cycles. This case highlights the potential benefits of Liu's scalp acupuncture therapy in enhancing motor function and overall neurological rehabilitation in children with cerebral palsy. Future research will focus on further validating this method and expanding its application to other developmental disorders.
Keywords: Liu's Scalp; Acupuncture Therapy; Cerebral Palsy
Cerebral palsy (CP) is a group of permanent movement disorders that emerge in early childhood, characterized by muscle stiffness or weakness, poor coordination, and tremors. These disorders primarily result from abnormal brain development or damage to the developing brain, either before, during, or shortly after birth. The global prevalence of CP ranges from 1.4‰ to 3.2‰, while in China, the prevalence among children aged 1-6 years is 2.46‰ [1-3]. The primary causes include prenatal factors such as congenital malformations and infections, perinatal factors like birth asphyxia and preterm birth, and postnatal factors including head trauma and infections [3,4].
Conventional treatments for CP typically involve a combination of physical therapy, occupational therapy, and medication. Physical therapy aims to improve motor skills and prevent contractures; occupational therapy focuses on enhancing daily living skills; and medications are used to manage symptoms such as spasticity and seizures [5,6]. Despite these interventions, many patients experience limited improvement, and the search for more effective treatment modalities continues [7]. The variability in treatment outcomes underscores the need for alternative or complementary therapies that can offer more substantial benefits.
Acupuncture, a traditional Chinese medicine technique, has gained attention as a potential treatment for neurological disorders, including CP. Liu’s scalp acupuncture therapy, developed by Professor Liu Zhenhuan, is a specialized form of acupuncture that targets specific areas of the scalp corresponding to brain regions involved in motor and sensory functions [8,9]. This case report examines the application and impact of Liu’s scalp acupuncture therapy on a pediatric patient with CP, highlighting its potential to enhance motor function and contribute to neurological rehabilitation. occupational therapy focuses on enhancing daily living skills;
The patient, a 2-year-old boy, was born on January 12, 2020, as the first of twins at 31 weeks and 2 days gestation via cesarean section, with a birth weight of 1 kg. He had a history of birth asphyxia and was hospitalized in the neonatal department for 19 days. The patient began treatment at our hospital on February 17, 2022, due to developmental delays. At 3 months old, the patient’s parents noticed that he could not lift his head, had poor responses, and exhibited deficient visual and auditory tracking. Growth and developmental milestones lagged compared to his peers. Multiple rehabilitation treatments at other hospitals yielded limited improvement. Initial assessment in our department showed the patient could lift his head, turn over, and stand with support but could not stand or walk independently. Past medical history revealed no significant findings other than the mentioned birth complications. The patient had no genetic disorders, infectious diseases, or history of surgeries or injuries. His developmental delays were evident in motor skills and neurological functions.
Professor Liu Zhenhuan developed the scalp acupuncture method used in this case, based on WHO standards and integrating techniques from various traditional acupuncture practices. This method targets specific cortical areas, such as the motor and pre-motor cortex, employing a unique needling technique aimed at stimulating neural rehabilitation and cognitive development. The treatment plan included main acupuncture points in the motor area, sensory area, foot motor-sensory area, and balance area 1, with supplementary points including the intelligence seven needles and temporal three needles. Treatment was administered every other day, with each session involving 10 needles. Each treatment course consisted of 30 sessions, followed by a 15-day rest period, and the patient underwent five treatment courses. Liu’s scalp acupuncture technique is characterized by a painless, rapid insertion method with an angle of 15-30° to avoid piercing the periosteum. The patient exhibited minimal discomfort and resistance during sessions, and his parents reported noticeable improvements in motor functions post-treatment.
After five treatment courses, the patient showed significant improvements in motor skills. He was able to stand and walk independently, although he exhibited a left-sided weight shift. He could kneel with both knees for over five minutes and walk on his knees for about three meters. His ability to transfer with moderate assistance was enhanced, and he could climb stairs with single-handed railing support. The Gross Motor Function Measure (GMFM-88) scores demonstrated marked improvement: 100 in lying and rolling, 98.3 in sitting, 90.5 in crawling and kneeling, 74.4 in standing, and 47.2 in walking, running, and jumping, resulting in a total score of 82.1 as of July 10, 2023.
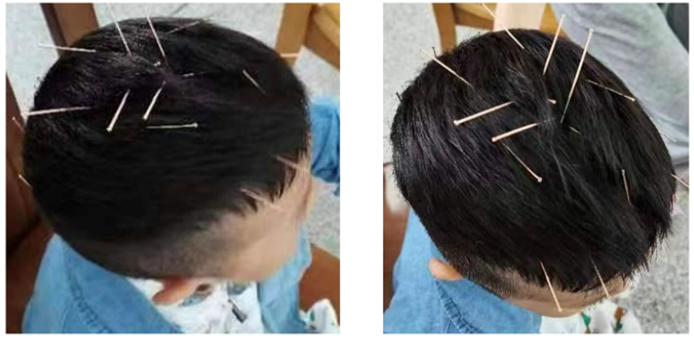
Figure 1: Application of Liu's scalp acupuncture therapy in treating the patient.
Liu’s scalp acupuncture therapy demonstrated significant benefits in this case, highlighting its potential as an effective treatment for CP. Key advantages of this approach include the precise localization of Brodmann’s cortical areas using tailored measurements and the enhanced effects of electro-acupuncture in critical cortical regions [8]. Integrating acupuncture with physical, speech, and occupational therapy maximized the patient’s outcomes [10,11]. This multi-modal approach leverages the synergistic effects of various therapies, resulting in more comprehensive and effective treatment. Additionally, the extended needle retention time is another critical factor contributing to the therapy’s effectiveness, as it allows for sustained stimulation of the target areas, promoting better therapeutic outcomes [8-11].
Functional MRI studies support the efficacy of scalp acupuncture in modulating cortical functions and promoting neural plasticity, further validating its role in neurorehabilitation [12]. Neuroplasticity, the brain’s ability to reorganize itself by forming new neural connections, is essential for recovery in CP patients [13]. Studies have shown that scalp acupuncture can enhance neural plasticity, facilitating the repair and regeneration of damaged neural pathways [14]. This case underscores the potential of Liu’s scalp acupuncture therapy as a valuable component of CP treatment, encouraging further research and integration of this technique into standard rehabilitation practices.
This case report highlights the promising potential of Liu’s scalp acupuncture therapy as an effective treatment for improving motor functions in children with cerebral palsy. The significant motor improvements observed in the patient, such as independent standing, walking, and enhanced coordination, point to the therapy’s efficacy. These positive outcomes suggest that Liu’s scalp acupuncture could be a valuable addition to the current therapeutic modalities for CP. The method’s innovative approach of combining precise acupuncture point targeting with extended needle retention time and integration with physical, speech, and occupational therapies appears to offer a comprehensive rehabilitation strategy. Future studies are warranted to further investigate and validate these findings, aiming to refine and standardize Liu’s scalp acupuncture technique for broader application. By conducting larger-scale clinical trials and exploring the underlying mechanisms through advanced neuroimaging techniques, this therapy could be optimized and widely adopted. This would potentially open new avenues for treating various pediatric neurodevelopmental disorders, ultimately enhancing the quality of life for affected children.
Copyright: © 2024 Alan Wang., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.