Vaibhav Oberoi1*, Inderjit Singh1 and Pooja Bhandari2
1Department of Neurology, Government Medical College, Amritsar, Punjab, India
2Department of Neurology, University of Mississippi Medical Center, Jackson, Mississippi, USA
*Corresponding Author: Vaibhav Oberoi, Department of Neurology, Government Medical College, Amritsar, Punjab, India.
Received: July 25, 2024; Published: September 25, 2024
Citation: Vaibhav Oberoi., et al. “An Unusual Case of Heavy Non-encephalitic Cysticercosis Presenting with Vertigo and Migraine: A Case Report”. Acta Scientific Neurology 7.10 (2024): 09-12.
Neurocysticercosis (NCC) is a parasitic infection caused by the larval stage of Taenia solium, predominantly affecting the central nervous system. We present a case study of a 45-year-old gentleman who initially presented with migraine-like headaches and later developed vertigo, ultimately diagnosed with disseminated intracranial NCC. Despite an initial diagnosis of migraine and treatment with symptomatic medications, persistent symptoms prompted further investigation including a 3D MRI, revealing multiple cysticercal lesions throughout the brain parenchyma. The patient was subsequently treated with albendazole and corticosteroids, leading to resolution of symptoms but later experienced a seizure necessitating antiepileptic therapy.
This case highlights the atypical clinical presentation of Neurocysticercosis with migraine-like headaches and vertigo, initially obscuring the underlying parasitic etiology. Recognition of Neurocysticercosis in regions where the disease is endemic, even without classical symptoms such as seizures, is crucial for timely diagnosis and management. Our report underscores the importance of comprehensive radiological evaluation in patients presenting with vertigo, especially in endemic regions, to avoid misdiagnosis and delay in appropriate treatment. Early initiation of anthelminthic therapy and adjunctive corticosteroids can effectively control symptoms and prevent complications like seizures. Further research and awareness are needed to enhance diagnostic strategies and optimize treatment outcomes for Neurocysticercosis globally.
Keywords: Cysticercosis; Neurocysticercosis; MRI
NCC- Neurocysticercosis; CNS: Central Nervous System; HIV: Human Immunodeficiency Virus; HBsAg: Hepatocellular B Virus Antigen; HCV: Hepatocellular C Virus; MRI: Magnetic Resonance Imaging; FLAIR: Fluid Attenuated Inversion Recovery
Cysticercosis is a condition caused by the larval stage (cysticerci) of the tapeworm Taenia solium. When this parasite infects the central nervous system, it causes neurocysticercosis (NCC) [1]. NCC is a prevalent parasitic disease in many developing countries and a significant curable cause of epilepsy in developing nations [2]. There is also a rising number of cases diagnosed in developed countries due to increased immigration from regions where the disease is endemic. Here, we present a case study of a middle-aged gentleman radiologically diagnosed with NCC which had disseminated to the entire brain parenchyma and presented with vertigo. Our objective is to broaden the clinical spectrum of the NCC and report a case of NCC with chronic atypical presentation of vertigo.
A 45-year-old gentleman presented to the outpatient department in November 2023 for the chief complaint of headache associated with nausea and vomit. The headache was unilateral (left temporoparietal area), pulsating in nature which was relieved with painkillers. The patient also suggested photo and phonophobia, resulting in a provisional diagnosis of Migraine made 6 months back. The patient was started on Naproxen 250 mg sos, and Valproate 250 twice daily as migraine prophylaxis. On follow up the patient explained decreased frequency and severity of the migraine episodes and the prophylactic dose of valproate was continued for 3 months. After 4 months, the patient presented again to the outpatient department with a chief complaint of vertigo. It was also associated with a persistent dull headache which was not relieved with the previous medications. The otological assessment revealed no signs of positional variation therefore ruling out Benign Paroxysmal Positional Vertigo. Lack of fever ruled out acute labyrinthitis. This new onset of vertigo and change in the pain characteristics prompted a 3D brain MRI. The brain MRI revealed multiple inflammatory well-defined T2/FLAIR hyperintense and hypointense nodular lesions with eccentric T2 hypointense scolex, diffusely scattered at gray-white matter in both cerebral hemispheres, basal ganglia, thalami, and bilateral cerebellar hemispheres suggestive of disseminated intracranial neurocysticercosis in varied stages (Figure 1: A, B). The electroencephalogram was normal, and the patient denied any history of abnormal body movement and seizure. The blood work was normal except for an HbA1C of 6 and random blood sugar of 150 mg/dl. The red blood cells were normochromic, normocytic, ruling out megaloblastic and iron deficiency anemia. The stool examination revealed no evidence of ova and parasites. The patient reported a history of significant weight loss in the past year. Chest X-ray was done to rule out any tuberculous foci. The X-ray skull was also normal. The blood serology showed non-reactivity for HIV, HBsAg and HCV. Serological assessment for toxoplasmosis was also negative. The neurological examination was normal with negative Romberg test, and signs of cerebellar disorder like, finger-to-nose test, dysdiadochokinesia, heel shin test, straight line walk test were absent. The general physical examination was negative for any kind of subcutaneous nodules in the muscle mass. The ocular fundoscopy and MRI spine were also negative for cysticercosis lesions.
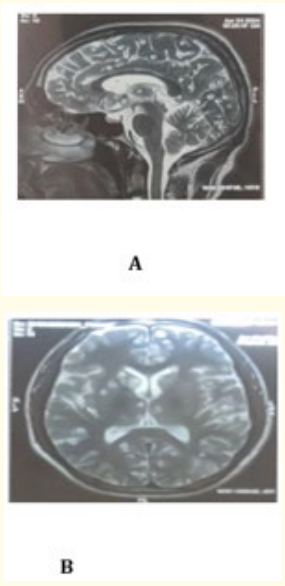
Figure 1: A, B. The brain MRI revealed multiple inflammatory well-defined T2/FLAIR hyperintense and hypointense nodular lesions with eccentric T2 hypointense scolex, diffusely scattered at gray-white matter in both cerebral hemispheres, basal ganglia, thalami, and bilateral cerebellar hemispheres suggestive of disseminated intracranial neurocysticercosis in varied stages in sagittal and axial sections.
Subsequently, the patient was started on albendazole 400 mg twice daily, with 6 mg of dexamethasone once daily along with betahistine 16 mg twice daily. The patient denied the inpatient admission and followed up regularly every week. Antiepileptics including Levetiracetam 500 mg and Sodium valproate 300 mg twice a day were also initiated. The patient was counseled about the inflammatory response that may trigger a seizure. In the second week of follow-up visit, the patient reported a seizure episode that lasted for 5-10 minutes, associated with generalized body movements, tongue biting, involuntary urination and postictal confusion. The neurologic examination was normal with mild dysarthria. The following day, 5 mg of Clobazam every night was added to the regimen. The patient reported no episodes of vertigo at the subsequent visits and has been seizure free since then. The albendazole and dexamethasone were stopped at 28 days and the patient continues antiepileptics including levetiracetam and valproate. A repeat MRI Brain is indicated but the patient refused further studies.
Neurocysticercosis (NCC) is a neurological infectious disease that occurs due to the infection of the central nervous system (CNS) by the larval stage of the pork tapeworm called Taenia solium [3]. NCC is widely prevalent in developing countries including Latin American countries, sub-Saharan Africa, and certain parts of Asia and represents the most common helminthic infection of the central nervous system. Conversely, NCC is uncommon in Northern Europe, the US, Canada, Australia, Japan, and New Zealand.
The life cycle of Taenia solium involves two hosts: humans and pigs. Pigs serve as intermediate hosts, while humans can act as both definitive and intermediate hosts. In most cases, human cysticercosis results from ingesting T. solium eggs directly from carriers (through fecal-oral contamination), and occasionally through self-infection via the fecal-oral route in adults harboring the adult parasite in the intestine. Once in the digestive tract, eggs develop into oncospheres, which travel through the bloodstream to the CNS and other tissues, where they mature into larval forms or cysticerci. Infected pigs play a role in transmitting taeniasis to humans but are not directly responsible for human cysticercosis [4].
The clinical symptoms of neurocysticercosis (NCC) vary depending on several factors: the number and location of lesions within the brain tissue, subarachnoid space, ventricular system, and spinal cord, as well as the strength of the immune response against the parasites. Patients with neurocysticercosis (NCC) usually present with seizures or epilepsy (occurring in approximately 70–75% of cases), headaches, focal neurological impairments, cognitive decline, or elevated intracranial pressure [5]. Most of the infected patients remain asymptomatic depending upon the stage of the lesion [6].
The treatment should be tailored based on the viability and location of cysticerci, as well as the intensity of the host’s immune response to the parasites. Primary management should prioritize controlling clinical symptoms (such as seizures, headache, and elevated intracranial pressure) and addressing underlying pathogenic mechanisms (such as brain swelling, inflammation, compressive effects, or hydrocephalus). This strategy involves the administration of antiepileptic drugs, anti-inflammatory agents, corticosteroids, corticosteroid-sparing medications either individually or in combination. Surgical interventions, including cyst removal, ventricular shunt placement, and decompressive craniotomy, may be necessary in some cases [1,7].
Recommended doses of albendazole typically range from 15 to 30 mg/kg/day, divided into two doses daily, over 8 to 10 days. A dosage of 15 mg/kg/day is adequate for parenchymal NC, whereas for extra parenchymal disease, a dosage of 30 mg/kg/day has demonstrated greater efficacy. Praziquantel (PZQ) has been used to treat human cysticercosis. Its exact mechanism against larvae is not fully understood. The recommended dose to treat NC patients is 50 mg/kg/day for 10 to 15 days. Two controlled studies performed by the same research group showed that the combination of both drugs (ABZ 15 mg/kg/day and PZQ 50 mg/kg/day for ten days) was significantly more efficient than ABZ alone in patients with three or more cysts [8].
Our 45-year-old gentleman presented with vertigo, episodes of spinning along with dizziness and migraine like headaches. A case report by Eliott., et al. described a 35 year old female who also presented with worsening migraines like headaches and a cystic brain lesion. Consequently, treatment with albendazole and dexamethasone led to the resolution of the symptoms and the lesion [9]. Fagong., et al. also reported a case of a 24-year-old man presenting with chronic headaches and was diagnosed as a case of NCC [10]. A report by Mesa., et al. reported a case of a 20-year-old gentleman presenting with vertigo and a cystic lesion in the fourth ventricle and compressing the brainstem [11]. Li., et al. reported a female with worsening dizziness over the past one year which was evidenced by an abnormal lesion in the spinal canal at the junction of the medulla oblongata and C1 that was causing hydrocephalus. The pathological examination on surgical removal revealed NCC [12].
Our patient reported to the outpatient department with chronic migraines and persistent vertigo. We ruled out benign paroxysmal positional vertigo as the dix hallpike maneuver was negative. There was no history of tinnitus, sensory neural hearing loss and fever therefore ruling out Acute Labyrinthitis and Meniere’s disease. The 3D MRI of the brain revealed multiple inflammatory well defined T2/FLAIR hyperintense and hypointense nodular lesions with eccentric T2 hypointense scolex, diffusely scattered at gray, white matter in both cerebral hemispheres, basal ganglia, thalami and bilateral cerebellar hemispheres suggestive of disseminated intracranial neurocysticercosis in varied stages, therefore confirming the diagnosis. [13] The treatment with albendazole and steroids has led to complete resolution of the symptoms, and he has been put on levetiracetam and valproate for seizure prophylaxis.
A repeat MRI brain may provide further insights into the disease resolution. However, due to patient non-compliance it cannot be executed.
We report this case because of the unusual presentation of NCC as migraine-like headaches and vertigo and no seizure. Through our case report we shed light on the atypical presentation of NCC and broaden the clinical spectrum of the disease. We also recommend thorough use of radiological investigations for patients experiencing vertigo in developing nations, along with careful consideration of antiepileptic drugs for preventing migraines that may mask seizure due to NCC. This approach will help diagnose this condition earlier, leading to a substantial decrease in patient stress and anxiety. It will also conserve valuable resources and time, which can be more efficiently allocated to treating other patients in need.
Copyright: © 2024 Vaibhav Oberoi., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.