Mohamed Khalil1, Ahmed El-Sadek2, Diaa Mostafa3, Asmaa Ibrahem4, Ahmed Ebied5, Asmaa Elballat6 and Tarek Youssif7*
1Specialist, Neurology Department, Phoenix Hospital, Abu Dhabi, UAE
2Assistant Professor, Neurology Department, Ain Shams University Cairo Egypt
3Specialist, Neurology Department, SEHA, Abu Dhabi, UAE
4Specialist, Neurology Department, Al-Raml Hospital Alexandria Egypt
5Lecturer, Neurology Department Faculty of medicine zagazig university, Egypt
6Specialist, Department of Neurology HMS garhoud hospital- Dubai- UAE
7Specialist, Neurology Department, Burjeel Holdings, Abu Dhabi, UAE
*Corresponding Author: Tarek Youssif, Specialist, Neurology Department, Burjeel Holdings, Abu Dhabi, UAE.
Received: July 27, 2024; Published: August 29, 2024
Citation: Tarek Youssif., et al. “Correlation Between Pulsatility Index and Functional Outcome Post-Intravenous Thrombolytic Therapy in Acute Ischemic Stroke; Multi-Centers Cases”. Acta Scientific Neurology 7.9 (2024): 15-20.
This study aimed to assess the Pulsatility index value as possible significant predictor of functional outcome among acute ischemic stroke patients who received intravenous thrombolytic therapy.
Patient and Methods: this is a case series report involved three cases from three different centers in Egypt and UAE, those patients had acute ischemic stroke and received intravenous thrombolytic therapy as per guidelines and they were followed up over a period of three months.
Results: in those patients the Pulsatility index value of same side of the lesion were assessed and correlated with their functional outcome using modified rankin scale and showed that the functional outcome was poor when PI value was increased.
Conclusion: PI value in the cerebral vessels at same side of stroke lesion is a promising significant predictor of functional outcome in patients with acute ischemic stroke who received thrombolytic therapy. Thus, we recommend further large studies involving large number of patients and explore it as a possible significant independent predictor of functional outcome.
Keywords: Pulsatility Index; Acute Ischemic Stroke; TCD
Stroke is the second most leading cause of death, and accounts for 11% of total world deaths in 2015 and the fifth leading cause of death in the United States, also more than 80% of strokes are ischemic strokes [1,2].
Pulsatility Index (PI) is increased in patients with vascular risk factors or pre-existing microangiopathy including hypertension, DM, retinopathy, nephropathy, and white matter disease [3,4]. These, insults lead to narrowing of cerebral small vessels and increase distal vascular resistance. This in turn causes the pattern of blood flow to be more pulsatile (increased systolic flow and decreased diastolic flow) in cerebral vessels, leading to a higher PI value on transcranial Doppler (TCD) assessment. Therefore, PI is regarded as a surrogate marker for cerebral small vessel disease [3].
PI has been also recognized as a marker of cerebral small vessel disease. PI is increased in those with other features of cerebral small vessel disease such as white matter disease and microbleed, which are established as poor prognostic markers in acute stroke by themselves. Higher PI may not only indicate a higher severity of small vessel disease but also, contribute to further vascular injury and progression of atherosclerosis in cerebral vasculature [5,6].
Male patient, 62-year-old, coronary artery disease (CAD), hypertensive, smoker, presented to emergency department (ED) complaining of right sided weakness, difficult articulation with intact repetition and comprehension, mouth deviation and numbness in the right side of the body 2 hours ago before presentation to ER, the condition started with sudden onset and stationary course. On examination: Fully conscious Glasgow come scale(GCS) 15/15, vitally stable (Blood pressure 140/90, temperature 37, pulse 80 beat/minute, respiratory Rate 20 breath/ minute), mild dysarthria, mild right upper motor neuron facial paralysis, right hemiparesis (lower limb more than upper limb, distal more than proximal, power in lower limb grade 3 and in upper limb grade 4), right hemi-hypoesthesia including face, right sided hyper-reflexes, right extensor plantar response, other neurological tests were intact, and the NIHSS score at time of admission was 8. Laboratory investigations were done, and no abnormalities detected, also CT brain was unremarkable study. The patient was eligible for intravenous(I)V thrombolysis according to AHA/ ASA (American heart association/American stroke association) guidelines, benefits and hazards of treatment were discussed with the patient and relatives, and written consent was obtained. The patient received IV thrombolysis at dose 0.9 mg/Kg, his body weight was 80 kg and received 72 mg (10% infused intravenously in 1 minute, the remaining 90% infused intravenously in 60 minutes) with door to needle time 50 minutes and onset to treatment time 170 minutes (less than 3 hours). Patient was carefully monitored (vitally, neurologically and generally) according to guidelines protocols during and after receiving the IV thrombolysis. After finishing IV thrombolysis the patient was reassessed and the NIHSS 1 hour after IV thrombolysis was 2, the patient was admitted to stroke unit to complete monitoring and stroke investigations work up, NIHSS 24 hours after IV thrombolysis was 0, CT brain follow up after 24 hours revealed acute left lacunar hypodense lesion in the posterior limb of internal capsule (as seen in figure 1), TCCD was done and PI in the left middle cerebral artery(MCA) was 0.86 (as seen in figure 2). According to TOAST classification, it was a stroke of undetermined etiology, and the patient was discharged to home after 72 hours, and NIHSS score was 0 and modified ranking score(mRs) was 0. The patient was followed up after 3 months of receiving IV thrombolysis and mRS was 0 (Good functional outcome).
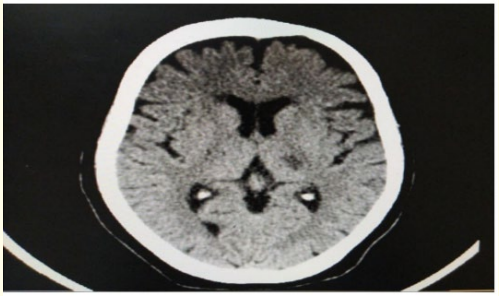
Figure 1: CT brain axial view follow up 24 hours after receiving IV thrombolysis showing acute left lacunar hypodense lesion in the posterior limb of internal capsule.
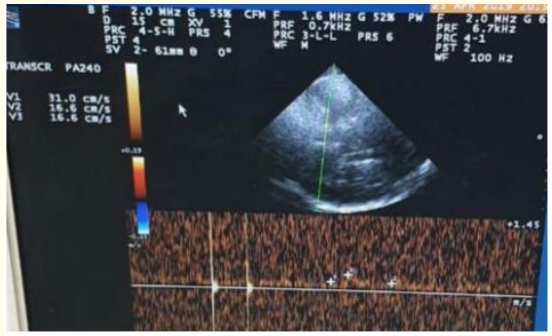
Figure 2: TCCD Transtemporal approach, Mesencephalic plane of left M1 middle cerebral artery (MCA), 24 hours after receiving IV thrombolysis showing normal PI (0.86) at the same side of the lesion.
Female patient, 70-year-old, atrial fibrillation (AF), type 2 diabetes mellitus (DM), presented to ED complaining of disturbed level of consciousness, left sided weakness, mouth deviation and numbness in the left side of the body and difficult speech, 90 minutes ago before presentation to ED, the condition started with sudden onset and stationary course. On examination: Disturbed conscious GCS 12/15, vitally stable (Blood pressure 130/80, Temperature 36.8, Pulse 90 beat/minute, Respiratory Rate 19 breath/minute), global aphasia, moderate left upper motor neuron facial paralysis, left hemiparesis (lower limb more than upper limb, distal more than proximal, power in lower limb grade 1 and in upper limb grade 2), left hemi-hypoesthesia including face, left sided hyperreflexia, left extensor plantar response, other neurological tests were intact, and the NIHSS score at time of admission was 16. Laboratory investigations were done, and no abnormalities detected, also CT brain was unremarkable study. The patient was eligible for IV thrombolysis according to AHA/ ASA guidelines, benefits and hazards of treatment were discussed with the patient and relatives, and written consent was obtained. The patient received IV thrombolysis at dose 0.9 mg/Kg, his body weight was 90 kg and received 81 mg (10% infused intravenously in 1 minute, the remaining 90% infused intravenously in 60 minutes) with door to needle time 45 minutes and onset to treatment time 135 minutes (less than 3 hours). Patient was carefully monitored (vitally, neurologically and generally) according to guidelines protocols during and after receiving the IV thrombolysis. After finishing IV thrombolysis the patient was reassessed and the NIHSS 1 hour after IV thrombolysis was 13, the patient was admitted to stroke unit to complete monitoring and stroke investigations work up, NIHSS 24 hours after IV thrombolysis was 10, CT brain follow up after 24 hours revealed acute right tempro-parietal Ischemic infraction (M2 MCA) (as seen in figure 3), TCCD was done and PI in the right MCA was 2.13(as seen in figure 4). According to TOAST classification, it was CE stroke, and the patient was discharged to home after 7 days, and NIHSS score was 10 and mRS was 4. The patient was followed up after 3 months of receiving IV thrombolysis and mRS was 3 (Poor functional outcome).
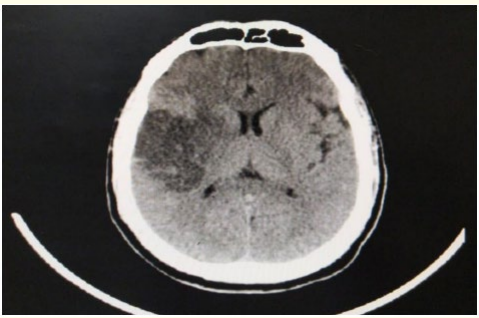
Figure 3: CT brain axial view follow up 24 hours after receiving IV thrombolysis revealing acute right tempro-parietal Ischemic infraction (M2 MCA).
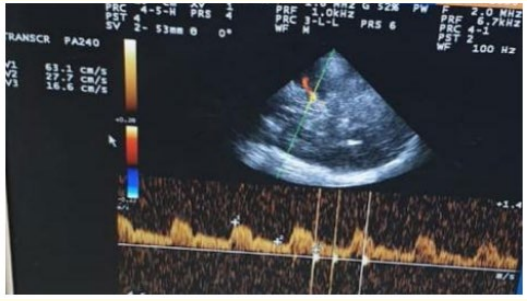
Figure 4: TCCD, Transtemporal approach, Mesencephalic plane of right MCA, 24 hours after receiving IV thrombolysis showing increased PI (2.13) at the same side of the lesion.
Female patient, 66-year-old, type 2 DM, presented to ED complaining of decreased level of consciousness, left sided weakness, 45 minutes ago before presentation to ED, the condition started with sudden onset and stationary course. On examination: Disturbed conscious GCS 12/15, vitally stable (Blood pressure 140/85, Temperature 36.8, Pulse 90 beat/minute, Respiratory Rate 19 breath/ minute), mild left upper motor neuron facial paralysis, left hemiparesis (lower limb more than upper limb, distal more than proximal, power in lower limb grade 2 and in upper limb grade 3),left homonymous hemianopia, left hemi-hypoesthesia including face, left sided hyperreflexia, left extensor plantar response, other neurological tests were intact, and the NIHSS score at time of admission was 13. Laboratory investigations were done, and no abnormalities detected, also CT brain was unremarkable study. The patient was eligible for IV thrombolysis according to AHA/ASA guidelines, benefits and hazards of treatment were discussed with the patient and relatives, and written consent was obtained. The patient received IV thrombolysis (Actilyse) total at dose 0.9 mg/Kg, his body weight was 100 kg, total dose 90 mg (10% infused intravenously in 1 minute, the remaining 90% infused intravenously in 60 minutes) with door to needle time 45 minutes and onset to treatment time 110 minutes (less than 2 hours). Patient was carefully monitored (vitally, neurologically and generally) according to guidelines protocols during and after receiving the IV thrombolysis. After finishing IV thrombolysis the patient was reassessed and the NIHSS 1 hour after IV thrombolysis was 10, the patient was admitted to stroke unit to complete monitoring and stroke investigations work up, NIHSS 24 hours after IV thrombolysis was 8, CT brain follow up after 24 hours revealed acute right temporooccipital infraction involving PCA territory (as seen in figure 5), TCCD was done and PI in the right PCA 2.13 (as seen in figure 6). According to TOAST classification, it was CE stroke, and the patient was discharged to home after 7 days, and NIHSS score was 8 and mRS was 3. The patient was followed up after 3 months of receiving IV thrombolysis and mRS was 3 (Poor functional outcome).
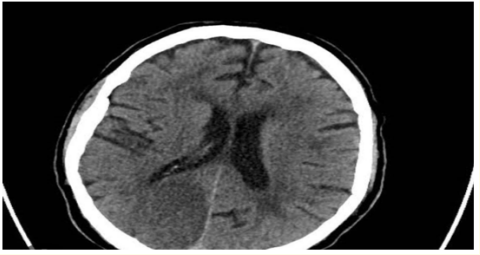
Figure 5: CT Brain without contrast axial view showing right tempro-occipital acute ischemic infarction involving right PCA territory.
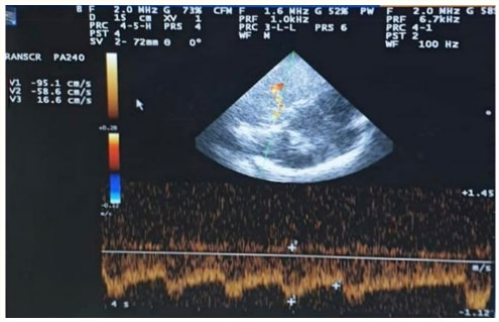
Figure 6: TCCD, Transtemporal approach, Mesencephalic plane of right PCA, 24 hours after receiving IV thrombolysis showing increased PI (2.13) at the same side of the lesion.
PI values are easily calculated from the waveform of blood flow in the cerebral artery examined and increases in accordance with distal vascular resistance and is considered a marker of small vessel disease and microangiopathic changes in brain10. These, insults lead to decrease size of cerebral small vessels and increase distal vascular resistance. This in turn causes the pattern of blood flow to be more pulsatile (increased systolic flow and decreased diastolic flow) in cerebral vessels, leading to a higher PI value on TCD assessment. Therefore, PI is regarded as a surrogate marker for cerebral small vessel disease [3].
In lacunar stroke, early hemodynamic factors are crucial in determining whether the hypo-perfused area will be turned into a permanent infarct [11]. PI is positively associated with elevation of intracranial pressure, which results in decreased cerebral perfusion pressure [12,13]. Also, the excessive pulsatile flow of cerebral circulation is accompanied by the decrease of total cerebral blood flow [11]. Under normal conditions, increased pulsatile flow could be compensated for by autoregulation of the cerebrovascular system.
However, as vascular reactivity is impaired in acute stroke, transmission of the increase in pulsatile flow may overcome the autoregulatory reserve, causing further brain damage [12]. Related to these, it was shown that segmental contraction occurs after the ischemia [13]. In addition, even though there is ischemia reperfusion in the early period, the capillary bed narrows segmentally by contraction of pericytes, and the passage of blood cells is prevented [14,15].
From these cases, it showed significant correlations between the increased value of PI and the poor functional outcome 3 months after IV thrombolysis and between the normal range value of PI and the good functional outcome 3 months after IV thrombolysis.
This agreed with previous studies like Nevzat., et al., 2013 [16] who explored the relationship between PI and clinical course of acute ischemic stroke after thrombolytic treatment and found a significant positive relationship between the NIHSS scores of patients at 24 hours and the PI that was measured on the recanalized MCA after IV thrombolysis and a significant positive relationship between mRS at 3 months and PI [17]. Also, Kim., et al., 2016 explored the effect of PI on infarct volume in acute lacunar stroke and demonstrated that PI was an independent determinant of infarct volume in acute lacunar stroke and consequently the functional outcome, and its measured value in acute stroke may be a surrogate marker of the extent of ischemic injury [17].
PI value in the cerebral vessels at same side of stroke lesion is a promising significant predictor of functional outcome in patients with acute ischemic stroke who received thrombolytic therapy. Thus, we recommend further large studies involving large number of patients and explore it as a possible significant independent predictor of functional outcome.
The study protocol was approved by the Ain Shams University, Faculty of Medicine Research Ethic Committee in addition to approval of ethical research committee in phoenix hospital Abu Dhabi and burjeel medical hospital. Participation was voluntary and all contributors or their first-degree relatives received detailed information about the aims of this research work and an informed consent was obtained prior to the commencement of the study.
A written informed consent for the publication was obtained from all the participants (or their first-degree relatives).
All authors declare that they have no competing interest.
Copyright: © 2024 Tarek Youssif., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.