Paul H Hartel1,2*, Antra Kamkovska3, Leah Quinn3 and Charlotte McQuillan3
1Sligo University Hospital, Ireland 2West Virginia University, USA 3Atlantic Technological University, Ireland
*Corresponding Author: Paul H Hartel, Professor, Sligo University Hospital, Ireland.
Received: August 30, 2024; Published: October 07, 2024
Citation: Paul H Hartel., et al. “Infection Related Desquamative Interstitial Pneumonia in Non-Smokers Discovered at Post Mortem Examination". Acta Scientific Microbiology 7.11 (2024):02-06.
We identified at post mortem examination, eight cases of histologic desquamative interstitial pneumonia (DIP) histologic pattern associated with current infection or signs of recent infection without the definitive fine hemosiderin pigment typically seen in DIP intraalveolar macrophages. We reviewed clinical and pathologic data from a total of 20 cases of DIP confirmed at autopsy. Of the eight cases negative for intraalveolar macrophage fine pigment, none was exposed to cigarette smoke or asbestos. Three had pulmonary infection with Candida (1), Streptococcal pneumonia (1) and Covid-19 (1). Five of the non-pigmented cases had indications of current or recent infection with inconspicuous organizing or acute pneumonia (4) and pulmonary necrotizing granulomas (1). All patients had ante-mortem symptoms of subjective respiratory distress or shortness of breath. None were HIV positive. DIP pattern has rarely been reported in conjunction with infection, and primarily only in HIV-positive patients. We report the first cases of infection-related DIP histologic pattern associated with predominantly immunocompetent patients, and common microbial infections.
Keywords: Desquamative Interstitial Pneumonia; Pulmonary Infection; Alveolar Macrophages
DIP: Desquamative Interstitial Pneumonia; HIV: Human Immunodeficiency Virus; CT: Computed Tomography; ED: Emergency Department; NF-1: Neurofibromatosis Type 1
Desquamative Interstitial Pneumonia (DIP) is a misnomer for an uncommon condition [1] with diffuse intraalveolar finely pigmented hemosiderin laden macrophages in lungs of persons exposed to cigarette smoke [2]. Other etiologies conventionally include asbestos exposure and presentation as an idiopathic entity [3]. We describe novel cases of DIP pattern in post mortem lung tissue associated with current or signs of recent pulmonary infection lacking the characteristic macrophage fine pigment and representing previously undescribed DIP. We propose new terminology to more accurately define this entity. Twenty post mortem cases were identified from 2015 through 2023 that included DIP pattern as a histologic finding. Clinical and histologic data were reviewed and included history of exposure to cigarette smoke or asbestos, presence or absence of intraalveolar macrophage pigment and primary diseases at autopsy. All cases met histologic criteria for DIP [3] showing uniform involvement of lung parenchyma with prominent accumulation of alveolar macrophages, along with absence of architectural remodeling with dense fibrosis, smooth muscle hyperplasia, fibroblast foci, conspicuous organizing pneumonia or extensive eosinophils. Patients were 11 males and 9 females ranging in age from 6-83 years (m = 57). Twelve cases showed intraalveolar macrophage fine pigment; 11 exposed to cigarette smoke and 1 with congestive heart failure. Eight were negative for intraalveolar macrophage fine pigment and three of these had pulmonary infection with Candida (1), Streptococcal pneumonia (1) and Covid-19 (1). Five of the non-pigmented cases had indications of current or recent infection with focal, inconspicuous organizing or acute pneumonia (4) and pulmonary necrotizing granulomas (1). All patients had ante-mortem symptoms of subjective respiratory distress or shortness of breath. The patient with pulmonary Candida infection had type II diabetes.
CoPath electronic archive search using keywords post mortem and desquamative interstitial pneumonia was performed. Twenty cases were identified from 2015 through 2023 and anonymized reports and hematoxylin and eosin and any available histochemical slides from these cases were reviewed by a pulmonary pathologist. Clinical and histologic data were compiled in an anonymized XL file and consisted of age, gender, exposure to cigarette smoke or asbestos, presence or absence of intraalveolar macrophage pigment, primary diseases at autopsy and ante-mortem symptomatology. All cases met histologic criteria for DIP [3] showing uniform involvement of lung parenchyma with prominent accumulation of alveolar macrophages in each case, along with absence of architectural remodeling with dense fibrosis, smooth muscle hyperplasia, fibroblast foci, conspicuous organizing pneumonia, extensive eosinophils, tumor and asbestos bodies.
Patients were 11 males and 9 females ranging in age from 6-83 years (m=57). Twelve of these DIP cases showed intraalveolar macrophage fine pigment (Figure 1); 11 exposed to cigarette smoke and 1 with congestive heart failure. Eight were negative for intraalveolar macrophage fine pigment (Figure 2) and three of these had pulmonary infection with Candida (1), Streptococcal pneumonia (1) and Covid-19 (1). Microorganisms were identified by tissue swab/culture and/or histochemistry in the latter three cases. Five of the non-pigmented cases had indications of current or recent infection without microorganisms identified, but with inconspicuous organizing or acute pneumonia (4) and pulmonary necrotizing granulomas (1). Prominent fibrosis, tumour, asbestos bodies or other pneumoconioses were not identified in any case. None of the decedants had a history of asbestos exposure or HIV infection. All cases reported antemortem respiratory distress or shortness of breath.
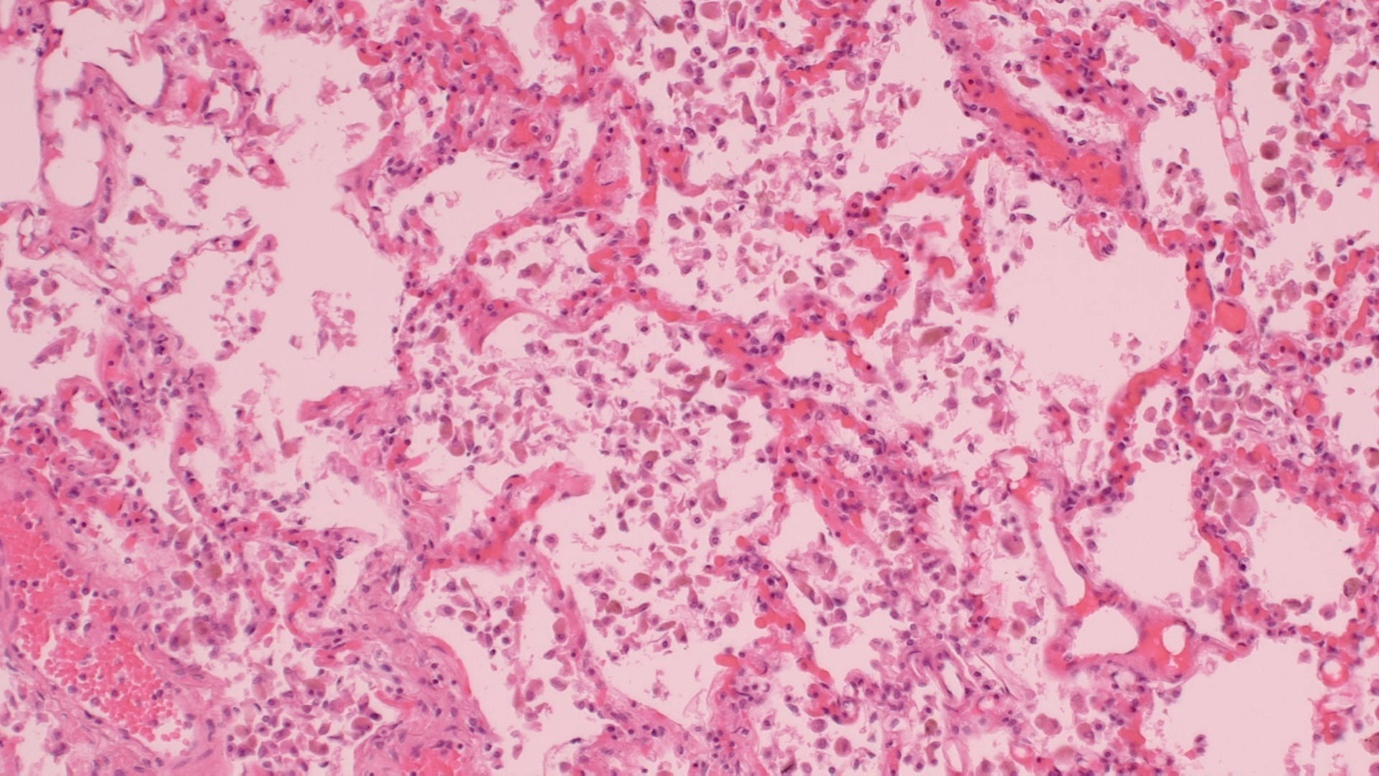
Figure 1: Classic DIP with finely pigmented hemosiderin laden macrophages (histiocytes), H&E stain, medium power.
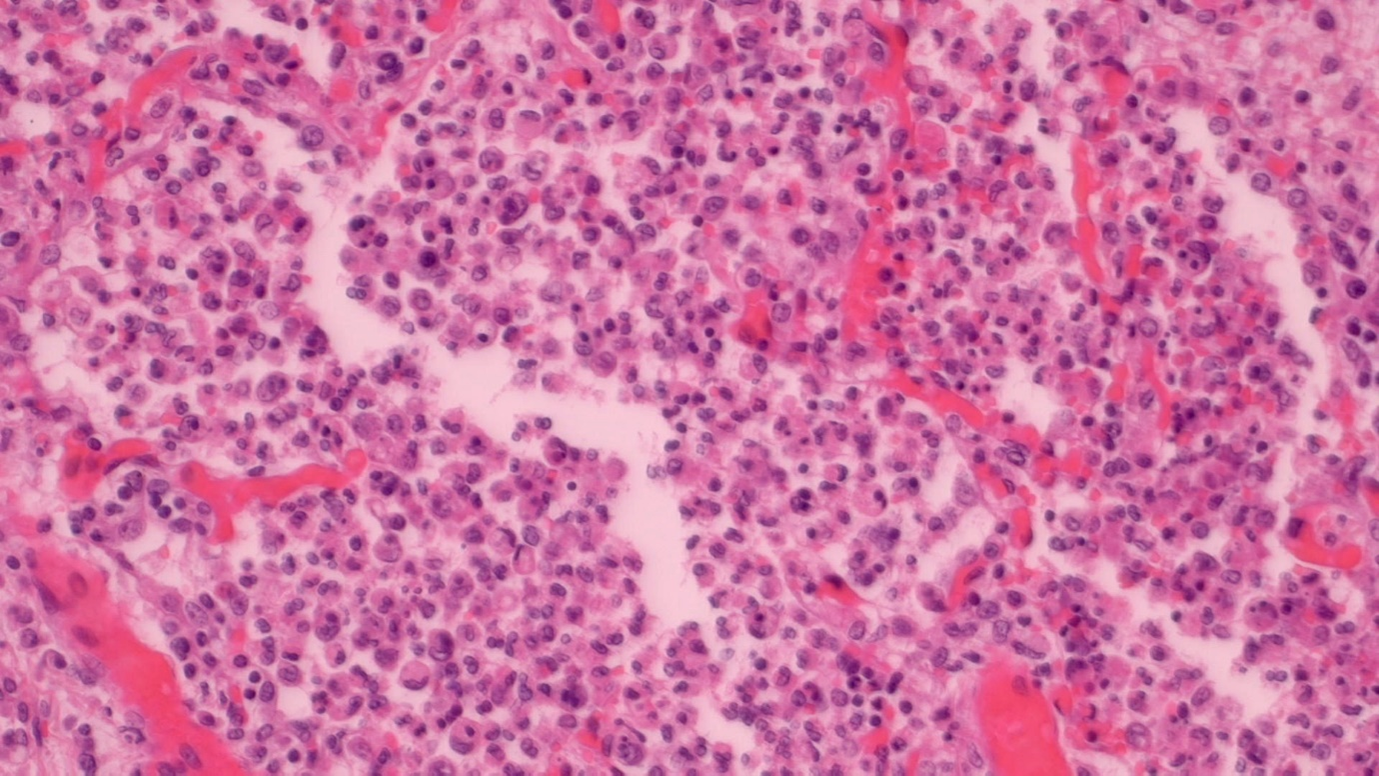
Figure 2: DIP with non-pigmented macrophages (histiocytes), H&E stain, medium power.
Desquamative interstitial pneumonia is conventionally a disease of smokers and less often those exposed to asbestos. It can also be an idiopathic condition. We identified DIP pattern in 20 post mortem cases of which eight had pathologic evidence of pulmonary infection or status post infection without history of cigarette smoke or asbestos exposure and a lack of the characteristic macrophage fine pigment seen in conventionally defined cases.
Desquamative interstitial pneumonia is defined as a clinical and pathologic entity found almost exclusively in current or former cigarette smokers. Other conventionally listed etiologies include asbestosis and presentation as an idiopathic entity. Desquamative interstitial pneumonia is characterised by diffuse macrophage infiltration of air spaces without prominent honeycombing or fibrosis. It was initially described by Liebow [2] in 1965 and the desquamated cells were thought to be epithelial in origin. The majority of intraalveolar cells in DIP were later shown to be macrophages [4]. Respiratory bronchiolitis shows similar histology although the macrophage infiltrate is focal and broncho/bronchiolocentric rather than diffuse as in DIP [3,5,6]. It has been previously suggested that a more technically correct term, such as macrophage alveolar pneumonia be utilised [7]. Clinically the incidence and prevalence of DIP are not known but represent from 3-8% of idiopathic interstitial pneumonias in specialised interstitial lung disease centres [8,9]. Radiologically high resolution CT shows bilateral symmetric ground glass opacification mainly in lower lung zones [10]. In approximately half of patients, a reticular pattern can be seen from focal, mild subpleural fibrosis in lower lung zones. Treatment is focused on smoking cessation and steroid therapy with an excellent prognosis compared with other interstitial pneumonias [11,12].
Desquamative Interstitial Pneumonia-pattern has been rarely described in macrophage-rich infections such as Mycobacterium avium intracellulare, Cryptococcus and Rhodococcus equi, particularly in HIV-infected patients [3]. However, this is the first report to our knowledge involving DIP pattern associated with more common infections, including Candida, Streptococcal pneumonia and Covid-19, in mostly immunocompetent patients. Rare case reports discuss DIP-pattern in other clinical settings. One involved a man in his early 40s with a history of a yearlong cough. He was a non-smoker but had a history of Neurofibromatosis type 1 (NF-1). He worked in information technology, had no environmental exposure to any smoke, toxic fumes or dust. Biopsy of his lungs showed they were hypercellular with increased, non-pigmented macrophages, consistent with a DIP-pattern diagnosis. Absence of any drug or environmental exposure suggest that this patient’s DIP may be associated with the NF-1 [13]. Another case involved a 27-year-old woman with an 18-month long history of a dry cough. She was a lifelong non-smoker and had no exposure to second hand smoke, asbestos or tuberculosis. She had however worked on a food production floor without a mask. Based on a clinical picture, CT findings and a biopsy carried out on her lungs she was diagnosed with DIP. Investigations into associations between DIP and workers in similar working environments could suggest a possible occupational etiology. There are previous cases of food production workers developing respiratory conditions from exposure to diacetyl [14]. A 59 year old female was admitted with dyspnoea, weakness, cough and a fever. A non-smoker, she had worked on a pig farm for 15 years before working with cleaning products and ironing. She was diagnosed with pneumonia. Probe based confocal laser endomicroscopy was performed, finding thickened intra-alveolar septum and a large number of auto fluorescent cells in alveoli [15]. Histological examination of surgical biopsy samples showed picture typical for DIP. A 13-month-old girl was admitted to the ER with acute shortness of breath, pulmonary bilateral ground-glass opacities and an almost complete opacification of left lung. She died 3hrs after admission and was diagnosed at autopsy with Covid-19, DIP as well as with an unusual IgA glomerulonephritis. Two other cases of DIP associated with chronic kidney failure in children were also discussed [16]. It is possible these cases were related to infection in immunocompromised patients. Finally, it has been espoused that there may be a link between DIP and having tattoos. A 29-year-old former smoker, with a large blue tattoo on his back visited hospital due to severe dyspnea. A lung biopsy was performed and revealed DIP. He died at aged 32, despite steroid therapy and smoking cessation. Tattoos are considered to be a foreign body which may incite pulmonary disease and DIP. However, further research is required [17].
Unfortunately, useful radiology imaging, specifically high resolution computed tomography was not available in any of our cases. It would be useful to compare radiologic findings from conventional smoking-related DIP with those of this sample. It would also be useful to know whether course of illness or prognosis differs between infection-associated DIP and conventional smoking-related cases. If in fact the course of illness associated with infection related DIP is mild and short-lived, then pulmonary biopsies are unlikely to occur in live patients. This may indeed be the case as DIP pattern associated with infections has not been previously reported other than in severely ill patients with HIV and opportunistic infections.
Contrary to conventional definition, we describe eight cases of DIP without cigarette smoke or asbestos exposure and lacking the typical macrophage fine hemosiderin pigment. None of the decedents had a history of HIV infection. We propose that more common and less severe pulmonary infection than those reported in HIV-infected patients can be associated with DIP pattern and suggest new terminology of ‘Diffuse Alveolar Histiocytosis, non-pigmented type‘ for these cases. Considering that our findings have not been previously reported in live patients, the course of illness and prognosis may be more benign than conventional DIP as lung biopsies are usually reserved for atypical or unwell patients. Although this is an uncommon condition, larger sample sizes are necessary for further and more detailed investigation to include imaging findings, course of illness and prognosis.
Copyright: © 2024 Paul H Hartel., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.