Asma E Elamari1*, Wafa S Zubi1, Tahani M Awin2, Nawal M. Buzgaia2 and Abdusslam M Elmogassapi1
1Botany Department, Science Faculty, University of Benghazi, Benghazi, Libya
2Chemistry Department, Science Faculty, University of Benghazi, Benghazi, Libya
*Corresponding Author: Asma E Elamari, Botany Department, Science Faculty, University of Benghazi, Benghazi, Libya.
Received: August 19, 2024; Published: September 24, 2024
Citation: Asma E Elamari., et al. “Antibacterial Activity of Tuber Extracts of Cyclamen rohlfsianum Against Some Human Pathogenic bacteria, In vitro". Acta Scientific Microbiology 7.10 (2024):49-54.
Cyclamen rohlfsianum is an endemic plant species to Al-jabal al-Akhdar, Libya. Lately, it got some attention in term of it’s antimicrobial activity and use to treat the infections that cause by bacteria and candida. This study investigated the antibacterial activity of Cyclamen rohlfsianum tuber extracts against four species of human pathogenic bacteria (Staphylococcus aureus, Pseudomonas aeruginosa, Enterobacter sp., and Escherichia coli) using disc diffusion method. Four solvents were used in the extraction process; which were hexane, chloroform, ethyl acetate, and methanol. The obtained results from all tuber extracts at concentration 0.01 mg/ml showed clear antibacterial activity against both S. aureus and P. aeruginosa. Ethanol tuber extract was the most effective against both S. aureus and P. aeruginosa (with inhibition zone 0.8 mm and 0.7 mm in diameter; respectively) compared to hexane, chloroform, and ethyl acetate tuber extracts. On the other hand, only chloroform plant extract inhibit the growth of Enterobacter sp. (with inhibition zone 0.5 mm in diameter). In addition, hexane and chloroform plant extract showed antibacterial activity against E. coli (with inhibition zone 0.3 and 0.5 mm in diameter, respectively) while the other applied plant extracts had no inhibitory activity against this bacterium. The study confirmed the efficacy of Cyclamen rohlfsianum tuber extracts as natural antibacterial and suggested that they may be used as a therapy to treat infectious diseases caused by the bacteria under study.
Keywords: Antibacterial Activity; Cyclamen rohlfsianum; Disc Diffusion Method
For many decades, scientists in medical field have been concern about explore new antimicrobial effective substances from different sources such as medicinal plants [9]. Plants have been used as sources of medicinal and today a significant proportion of the population in the developed and developing countries depends on herbal medicines, especially in the areas where infectious diseases are endemic and modern health care facilities are unavailable.
In Libya, many people are consider traditional system of medicine as a first choice before visiting health care center that may be because of the fact that traditional medicine is safer and not costly [18]. That encourages the scientific community to incorporate the plants as very important part of the researches that concern about human health. Many studies have been reported that plants contain many biologically active compounds that affect the growth of human pathogenic microorganisms [4]. That leads to discover newer, safer, and possibly more effective drugs against human pathogenic bacteria and fungi.
Cyclamen rohlfsianum is one of the medicinal plants that belong to the family Primulaceae. It is native to North Africa, and it is one of the tenderest cyclamen species. A tetraploid Cyclamen rohlfsianum is endemic to Al-Jabal Al-Akhdar [6,16], and it is locally known as “Racuf” [8,22]. It is well known that Cyclamen rohlfsianum is a poisonous plant because it contains cyclamen glycoside [23] but it still commonly use in the therapy of diabetes though it is a toxic plant [7]. Many reports indicated that Cyclamen sp. contains active compounds such as alkaloid, flavinoid, phenolic compound, terpenoid, and saponins that may be attributed to its effect on the growth of human pathogenic bacteria and fungi [30-33]. In addition, many studies have been showed that Cyclamen spp. had antimicrobial activity against wide variety of human pathogenic bacteria and candida [7,13]. Moreover, many studies have indicated the antimicrobial effect of Cyclamen persicum tuber and leaves extracts against some of human pathogenic bacteria and candida; such as Escherichia coli, Staphylococcus aureus, Proteus vulgaris, Pseudomonas aeruginosa, and Candida spp. [2,10,13] Therefore, the main goal of the current study is to evaluate the antimicrobial activity of tuber extracts of Cyclamen rohlfsianum against some human pathogenic bacteria.
Fresh samples of Cyclamen rohlfsianum (Family- Primulaceae) were collected from Al-jabal al- Akhdar, Libya in March 2023. The plant was identified and classified at Department of Botany, University of Benghazi. The collected plants were transported to the laboratory and washed with water and dried in the shade for 14 days. The dried tubers were separated from each plant and were crushed into a fine powder using an electric blender [21]. 50g of the powder of Cyclamen rohlfsianum tubers was filled in the thimble and extracted with 100 ml of each solvent (hexane, chloroform, ethyl acetate, and methanol; separately) using a Soxhlet apparatus under controlled temperature for 24 hours then all the extracts were evaporated. Finally, all the crude extracts were dissolved in the 1% (v/v) dimethyl sulphoxide (DMSO) [2]. Only one concentration of the extracts was prepared (0.01 mg/ml) and stored at 4 °C in airtight bottles until further use.
Bacterial species were obtained from the microbiology laboratory of Children Hospital, Benghazi, Libya. Four human pathogenic bacteria; which are Staphylococcus aureus, Pseudomonas aeruginosa, Enterobacter sp. and Escherichia coli of clinical importance were used in this study. Bacteria were identified using BD Phoenix for accurate identification. The isolates were cultured in nutrient agar (NA) at 37 °C and were maintained on nutrient agar slants at 4 °C.
Bacterial stock cultures were sub-cultured onto nutrient agar (NA) plates and incubated overnight at 37°C. Three to four bacterial colonies were inoculated into 10 ml of Mueller Hinton Broth (MHB) and incubated at 37 °C then the overnight bacterial suspensions were diluted and adjusted and applied [27].
For the detection of antibacterial activities the disc diffusion method (Kirby-Bauer antibiotic testing) was followed with modification [17]. Mueller-Hinton Agar (MHA) was used as basal medium for culture of bacteria (MHA was prepared by suspending 38 g in 1000 ml of distilled water). The medium was sterilized by autoclaving at 15 lbs pressure and 121°C for 15 minutes, cooled to 45-50°C and then 15 ml of the sterilized medium was poured into sterile petri plates. After solidification, 100 μl of each prepared isolate was inoculated on MHA plates using sterilized spreaders. Gentamycin (10 μg) was applied as a positive control and DMSO was applied as negative control (DMSO would not affect the growth of bacteria [1]. Sterile discs were filled with (20μl) of each extract (at the prepared concentration (0.01 mg/ml) and allowed to diffuse at room temperature for 1 hour then the plates were incubated at 37 °C for 24h. Triplets of the experiment were maintained for each bacterial species to ensure reliability. Finally after incubation time the diameter of the inhibitory zones formed around each disc were measured in mm and recorded.
The experiments were designed according to the complete random design. Statistical analysis was performed using Minitab 17 program and ANOVA variance analysis tables. The averages were compared using Tukey's test at P <0.05.
The results from the experiments were showed in table (1) and figure (1). In this study, Cyclamen rohlfsianum was investigated to evaluate its antibacterial activity against four species of human pathogenic bacteria, which are Staphylococcus arueus, Pseudomonas aeruginosa, Enterobacter sp. and Escherichia coli using a disc diffusion method. Gentamycin (10 μg) was applied as a positive control and DMSO was applied as negative control for the antibacterial assays.
In general, the study showed that all used tuber extracts at concentration 0.01 mg/ml exhibited a varying degree of antimicrobial activity against all bacteria tested (table 1). Hexane, chloroform, ethyl acetate, and ethanol extracts of the tubers of Cyclamen rohlfsianum showed inhibitory effect against both Staphylococcus aureus and Pseudomonas aeruginosa. Ethanol extract was the most effective plant extract against Staphylococcus aureus and Pseudomonas aeruginosa (with inhibition zone 0.8 and 0.7 mm in diameter; respectively) compared to the other extracts (hexane, chloroform, and ethyl acetate extracts).On the other hand, both ethanol and ethyl acetate extracts of the used plant had no effect on the growth on both Enterobacter sp. and Escherichia coli. Also, the results of this study showed that only chloroform tuber extract affect the growth of Enterobacter sp. (with inhibition zone 0.5 mm in diameter). In addition, both hexane and chloroform plant extract affect the growth of Escherichia coli (with inhibition zone 0.3 and 0.5 mm in diameter, respectively). However, ethyl acetate and ethanol plant extracts did not record any inhibitory activity against E. coli.
The results also showed the extent of an effect of the antibiotic used (Gentamycin) on the growth of the bacteria under study where it affected the growth of S. aureus, P. aeruginosa, Enterobacter sp. and E.coli (with inhibition zone 0.1, 0.1, 0.7, and 0.6 mm in diameter; respectively). It was clear that E. coli was the most resistant to both tuber extracts and gentamycin while S. aureus and P. aeruginosa were the most sensitive to both of them. In general, the comparison between the obtained results from tuber extracts and the antibiotic applied showed that the antibiotic had higher antibacterial activity than the tuber extracts against the growth of the tested bacteria but the plant extracts still affected the growth of the bacteria. Finally, the results showed that there were significant differences of the effect of different tuber extracts (hexane, chloroform, ethyl acetate, and ethanol) on the growth of all used bacteria P < 0.05.
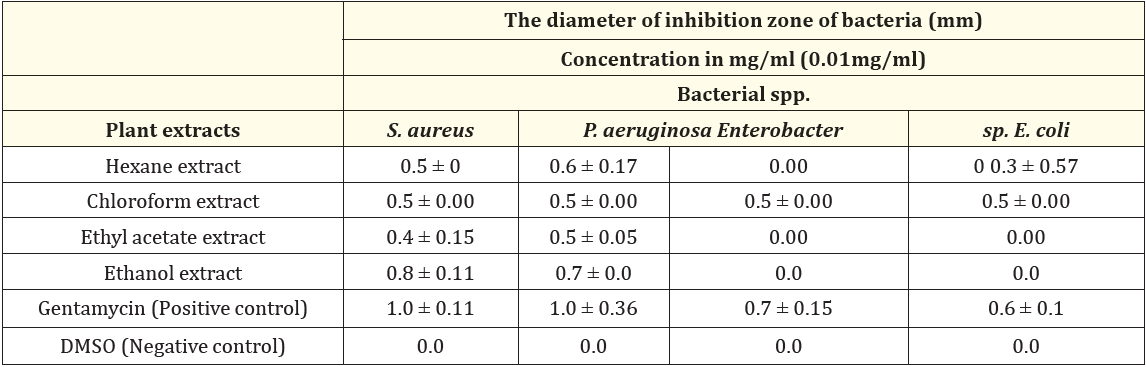
Table 1: Antibacterial activity of Cyclamen rohlfsianum tuber extracts against tested bacterial species.
(0.00): No inhibition zone
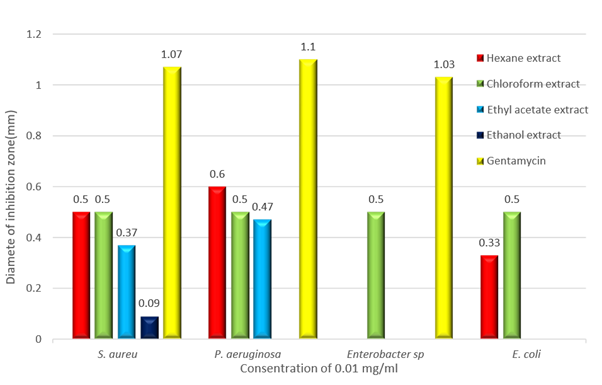
Figure 1: Effects of hexane, chloroform, ethyl acetate, and methanol plant extracts against tested bacterial species.
Due to the medical importance of Cyclamen rohlfsianum and its commonly used in alternative medicine in Libya, researchers pay attention of this plant [13]. In Libya, there is lack of research on the biological activities of endemic plants, therefore this study was conducted. The results showed that Cyclamen rohlfsianum tubers extracts had inhibitory effect against the growth of both Gram- positive and Gram-negative bacteria under study and this result agreed with [2]. In addition, the results agreed with the study which indicated that Cyclamen persicum tuber extracts had inhibitory effect on the growth of some bacteria and candida species [13]. Moreover, another study where showed that aqueous extract of leaves and tubers of Cyclamen rohlfsianum affect the growth of Escherichia coli, Staphylococcus aureus, Proteus vulgaris, and Pseudomonas aeruginosa, and that agree with our results [2].
The results also agreed with the study that indicated that aqueous extract of tubers of Cyclamen purpurascens L. had a potential effect on the growth of some types of human pathogenic bacteria [27]. In addition, several studies have shown that the extracts obtained from the Cyclamen species inhibit the growth of many species of bacteria and candida at various concentrations [2,3,29]. In general, many reports indicated that plants contain some active compounds such as flavonoids, aldehydes, ketones, saponins, alcohols, and phenolic compounds that inhibit the growth of microorganisms such as bacteria and candida [30,32,33]. and it is well known that these compounds are abundant in the Primulaceae family that Cyclamen rohlfsianum belongs to [3,7].
In common, our results showed that Gram-negative bacteria were more resistant to the tuber extracts and it may because the outer-membrane permeability barrier limits access of the antibacterial agents to their targets in the bacterial cell [9,12]. The active metabolic components of plant extracts such as terpenoid, alkaloid, and phenolics bind with enzymes and proteins found in bacterial cell membrane that lead to cell death or may inhibit the activity of the enzymes needed for amino acid biosynthesis [24,35]. The results showed that Enterobacter sp. was the most resistant to the plant extract and only chloroform plant extract inhibit the growth of this bacterium with inhibition zone 0.5 mm in diameter. In contrast, S. aureus was the most sensitive to both plant extracts and gentamycin. The Inhibitory effect of Cyclamen rohlfsianum could be refed to present active metabolites such as alkaloid, flavinoid, phenolic compound, terpenoid, and saponins as what said early. Therefore this research would recommend the importance of Cyclamen rohlfsianum for pharmacological use and to continue researches for purifying the active ingredient and to be subjected to furthermore standardized drug assays.
Cyclamen rohlfsianum tuber extracts inhibit the growth of some important human pathogenic bacteria, and it may be attributed to present of alkaloid, flavinoid, phenolic compound, terpenoid, and saponins. Therefore, this research recommends conducting more studies on the endemic Libyan plants in term of detection and purification of the active compounds and in order to incorporate these plants in the treatment of the infections caused by such bacteria.
Copyright: © 2024 Asma E Elamari., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.