Ikpeama Roseanne Adah1*, Ndefrekeabasi Itek Robinson2, Stepenson Danagogo Lawson2, Nyenke Clement Ugochukwu1 and Onosakponome Evelyn Orevaoghene1
1Department of Medical Microbiology, PAMO University of Medical Sciences, Port Harcourt, Rivers State
2Department of Medical Microbiology/Parasitology, Rivers State University, Port Harcourt, Rivers State
*Corresponding Author:Ikpeama Roseanne Adah, Department of Medical Microbiology, PAMO University of Medical Sciences, Port Harcourt, Rivers State.
Received: July 19, 2024; Published: September 11, 2024
Citation: Ikpeama Roseanne Adah., et al. “Chancroid: A Painful Sexually Transmitted Infection with Effective Treatment". Acta Scientific Microbiology 7.10 (2024):09-16.
A sexually transmitted infection known as Chancroid is caused by a bacterium Haemophilus ducreyi. It presents as a very painful ulcer which is mostly accompanied with a swollen lyphnodes in the groin. It is still found in many parts of the world. The illness initially manifests as a tiny inflammatory lesion that develops into an excruciating ulcer. Clinical presentation and laboratory testing utilizing culture, serology, and Polymerase Chain Reaction (PCR) technique to confirm the infection are the basis for the diagnosis of chancroid. Antibiotics are used as part of treatment and human immunodeficiency virus patients need longer courses of therapy. Promoting safer sexual practices, such as consistently using condoms, early diagnosis, and treatment to stop the disease's spread and lower the risk of HIV transmission are parts of prevention strategies.
Keywords: Chancroid; Haemophilus; Ulcer; Sexually
Chancroid continues to be a major focus of public health initiatives aimed at reducing the burden of Human immunodeficiency virus/acquired immunodeficiency syndrome and controlling sexually transmitted infections because of its connection to the sex industry and ability to spread Human immunodeficiency virus.
The bacterium Haemophilus ducreyi is the etiological agent of the sexually transmitted infection (STI) known as chancroid [1]. Swollen lymphnodes in the groin and excruciating genital ulcers are the resultant effects of the infection. Non-genital cutaneous ulcers are thought to be caused by Haemophilus ducreyi, and these ulcers typically affect children in tropical regions such as Africa, Asia, Latin America, the Caribbean and parts of the United States. Chancroid is more prevalent in developing nations and is frequently connected to sex workers for hire. It does not have an animal host or intermediate environment but pathogenic only in humans. The risk of Human immunodeficiency virus (HIV) transmission is increased by chancroid disruption of the mucosal barrier, which makes it a serious public health concern, particularly in areas with high HIV prevalence. The incubation period is generally 4-10 days but individual experiences may vary.
It is defined by painful, open, necrotizing vaginal ulcers or sores; inguinal lymphadenopathy, enlarged lymphnodes in the groin region may also accompany the condition [2]. The ulcers are often filled with pus, have soft edges, and feel uneven to the touch. Despite being highly transmissible, the disease is curable [3]. According to [4], skin-to-skin contact with open wounds or sores is typically how the infection is spread. The infection starts as a papule, quickly progresses to a pustule and finally becomes an ulcer [5].
The illness is linked to a higher risk of HIV infection and can lead to serious complications [6]. Painful vaginal ulcers are used to diagnose chancroid, which is treatable with the right antibiotic regimen [7]. Using barrier protection during sexual activity and adopting safe sexual practices are key components of chancroid prevention.
Haemophilus ducreyi is a facultative anaerobic coccobacillus that is small, gram-negative, and highly contagious. Being facultative means that H. ducreyi can survive in both oxygen rich and oxygen poor environment. While it is uncommon in the United States, it is a common cause of genital ulcers in Africa [8]. There is no intermediate environmental host or animal host; it is exclusively pathogenic in humans. The disease is extremely contagious and can cause painful vaginal sores as well as enlarged lymphnodes in the groin area. The ulcers have rough or worn edges and feel soft to the touch. About 25% of cases result in suppurative bubo development, which can progress to spontaneous rupture and the development of a deep, non-healing inguinal ulcer if left untreated [9].
In the Asia Pacific area, chronic limb ulcers have recently led to the isolation of Haemophilus ducreyi, the etiologic agent of chancroid ulcers [10]. Haemophilus ducreyi is a common cause of non-genital cutaneous ulcers, primarily affecting children in certain regions. It is also thought to be the cause of chronic limb ulcers in endemic area. With a median duration of 5-7 days, the incubation period ranges from one day to two weeks. The condition frequently begins as a mild inflammatory lesion and progresses to a painful genital ulcer. The size of the ulcer largely dictates how long it takes to heal; larger ulcers may take longer than two weeks [11]. When an ulcer is beneath the foreskin in an uncircumcised man, healing takes longer. Adequate antibiotics can easily treat the condition, but longer therapy rounds are necessary for those infected with HIV due to potential interactions and slower healing processes. After one to three months, the genital lesion goes away on its own if treatment is not received. Untreated infection, however, can worsen and result in severe inguinal lymphadenopathy, which in 25% of cases can ulcerate and result in buboes. Effective chancroid antimicrobial therapy eliminates the infection, treats the clinical symptoms, and stops the infection from spreading to other people [11].
Chancroid was estimated to afflict 7 million people globally in the 1990s, especially in low-resource countries in Africa, Asia, Latin America, and the Caribbean [12]. Nonetheless, data spanning from the 1980s to 2014 demonstrated a noteworthy decrease in the percentage of chancroid across all regions examined. Painful, purulent, deep ulcers with jagged, undermined edges are the hallmark of chancroid. The appearance of the ulcer is frequently used for diagnosis, though laboratory testing may be necessary [12]. Men are more likely to have the disease than women, with uncircumcised men experiencing it more frequently than circumcised people [13]. In the United States and globally, chancroid is considered an extremely rare sexually transmitted infection (STI), making up an ever-smaller fraction of all cases of genital ulcer disease. The age group most affected is 21–30 years old, with the highest prevalence among US women being 15-19 years old [9]. There are conflicting reports about the prevalence of chancroid genital ulcers in Nigeria; the country lacks adequate epidemiological data on the condition.
Significance as an Infection Transmitted by Sexual Contact. Because chancroid ulcers are linked to a higher risk of contracting HIV, they are significant as a sexually transmitted infection (STI). Genital sores, including those caused by chancroid can increase the risk of HIV infection by providing a route of entry for the virus [14]. Additionally, it has been found that chancroid plays a significant role in the heterosexual acquisition and transmission of HIV [15]. In order to stop the spread of chancroid and lower the risk of contracting HIV, patient education and the use of barrier protection during sexual activity, such as condoms, are crucial [16].
Due to its potential impact on public health and its association with HIV and other STIs, chancroid represents a decreasing percentage of all cases of genital ulcer disease; therefore, it is imperative to diagnose and manage the condition.
Chancroid was once widespread in many areas of the world, it has been completely eradicated as an endemic disease in industrialized nations due to concerted efforts to raise social awareness, changes in sexual behavior and improved diagnosis and treatment options [12]. The actual incidence of chancroid is unknown worldwide, but it has significantly decreased in nations like Thailand, Senegal, the Philippines, and India. This decline may be attributable to cooperative initiatives against STIs and the HIV/AIDS virus. Chancroid is extremely low in the United States with only a few cases reported annually [12].
The percentage of chancroid among genital ulcerative diseases (GUD) dropped from 69% to 15% in 2000 [17]. It is still common in some developing nations, including those in Asia, Africa, and the Caribbean [17]. Joint STI/HIV control programs do exist, but prevention and control strategies have not been consistently applied [18]. Travelers to these high-risk areas run the risk of catching the disease because outbreaks among sex workers also happen in these cities. Furthermore, outbreaks in the industrialized world are still caused by people from high-risk areas who travel abroad to work in the sex industry [17]. According to [19], ulcerative STIs cause tissue damage and ulceration by penetrating the skin of the external genitalia, colonizing the subcutaneous tissue, and causing ulceration. The normal skin must be penetrated by skin abrasion and microtrauma. HIV is more likely to enter the bloodstream and infect inflammatory cells when the mucosal barrier weakens, acting as a focal point for bacterial and viral shedding [20]. The World Health Organization (WHO) estimates that ulcerative STIs raise the risk of HIV transmission by 10%-50% for women and 50%-300% for men, [21]. HIV shedding is more likely in cases of multiple genital ulcers, purulent ulcer bases, and multiple genital ulcerative lesions [22].
The pathogenesis of chancroid involves the bacteria penetrating the skin and mucosal barrier, causing tissue damage and ulcers [23]. The rupture of the mucosal barrier increases the chance of HIV entering the bloodstream, and chancroid has been identified as a key cofactor in the heterosexual acquisition and transmission of HIV [24]. The ulcers are often soft to the touch and can be extremely painful, and regional lymphadenopathy occurs in around 50% of infections [25]. About 50% of infections result in regional lymphadenopathy, and the ulcers are frequently soft to the touch and extremely painful. Erythematous papules, or reddish lumps, are the first sign of the immunopathogenesis of Haemophilus ducreyi infection. These lumps eventually develop into pustules and ulcers, which cause excruciating ulcers and suppurative lymphadenopathy [26]. Seldom do patients seek medical assistance until they have ulcers for one to three weeks, typically three to six weeks following vaccination. The disruption of the mucosa in genital ulcer disease creates a portal of entry for HIV, increasing the risk of infection. There is a high likelihood that an infected individual will spread the disease during a single sexual encounter. When skin-to-skin contact occurs with open wounds, the bacteria (H. ducreyi) spreads and causes a localized inflammatory response [27]. Its detrimental effects seem to be caused by a cytocidal distending toxin that it produces. Patients need to be informed about the dangers of reinfection, how to use condoms as a barrier protection during sexual activity, and the importance of seeking medical attention for diagnosis and treatment [27].
Although chancroid ulcers can look different, they typically have the following traits
One or more painful genital sores may develop as a result of chancroid. These are soft to the touch ulcers, unlike those caused by syphilis, which are hard or rubbery. Chancroid ulcers typically have eroded or jagged edges, soft borders, and are painful and red. They are more common in males who have not had their periods and may leak pus. Pain and swelling in lymphnodes: Lymphadenopathy, or swelling and pain in the lymph nodes of the groin, is a common side effect of chancroid ulcers. It is common to see inguinal buboes in the groin or swollen, painful lymph glands. Regional lymphadenopathy is associated with about half of cases of chancroid. Women with chancroid may not exhibit any symptoms at all or may not even be aware that they have a lesion. Additional manifestations: [1] reported that chancroid can also appear as pseudo granuloma inguinale, follicular chancroid, giant chancroid, dwarf chancroid, and transient chancroid. Bubbo, or unilateral painful inguinal lymphadenopathy, is sometimes associated with chancroid ulcers. A diagnosis of Chancroid is indicated by the presence of one or more painful genital ulcers and tender suppurative inguinal adenopathy [1]. Almost half of patients experience painful inguinal lymphadenopathy within 1-2 weeks, which eventually leads to unilateral ulceration. Ulceration could result from a suppurating lymph node that drains into the skin. The ulcers typically have eroded or jagged edges, soft margins, and are painful and red.
They are more common in males who have not had their periods and may leak pus. In contrast to a syphilitic chancre, which is hard or rubbery to the touch, the ulcers are soft. According to [1], painful lymphnodes that often correspond with chancroid can develop in the groin. Chancroid ulcer symptoms include the following: Chancroid causes painful genital ulcers that are frequently accompanied by unilateral tender inguinal lymphadenitis (bubo). Urination or sexual activity pain: People with Chancroid may feel pain when urinating or having sexual activity. Groin swelling: At the junction of the thigh and lower abdomen, the groin can swell as a result of chancroid. Swollen lymph nodes: Chancroid therapy may result in swollen lymphnodes in the groin area, which may burst through the skin to cause sizable draining abscesses.
Bleeding and infectious fluid: If an ulcer is touched, it may bleed readily and release an infectious fluid that can transfer bacteria through the intercourse.
Bleeding and infectious fluid: Nearly half of the affected individuals will develop tender regional lymphadenopathy; women may experience nonspecific symptoms like painful urination, vaginal discharge, and rectal bleeding; some individuals may experience mild symptoms or be asymptomatic particularly women [1]. Tenderness and contagious fluid: The ulcers may spill easily if touched and produce a contagious fluid that can spread bacteria through intercourse.
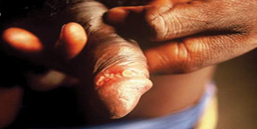
Figure 1
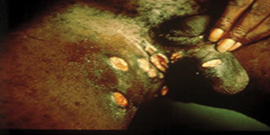
Figure 2
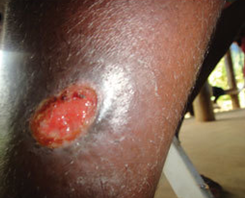
Figure 3
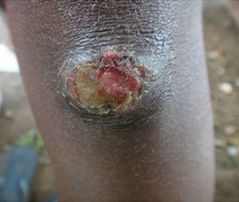
Figure 4
Diagnosis is primarily based on clinical presentation and confirmed with laboratory tests such as culture and PCR. Clinical and surveillance criteria, such as the existence of painful genital ulcers, the lack of Treponema pallidum infection, the typical appearance of genital ulcers, and a negative HSV test on the ulcer exudate, can be used to make the diagnosis. A combination of the patient's history, laboratory tests, and clinical presentation are used to diagnose chancroid ulcers. Important diagnostic markers for chancroid include the existence of a painful genital ulcer, regional lymphadenopathy, and a history of sexual exposure. Based on the appearance of the ulcer, which is typically deep, painful, purulent, and with uneven undermined edges, chancroid is diagnosed clinically. To diagnose chancroid ulcer in a laboratory setting, the bacteria Haemophilus ducreyi is isolated from a genital ulcer culture [28] using chocolate agar medium as well as a Gram stain of the ulcer swab [1]. Laboratory confirmation using culture and PCR is usually necessary because serologic detection and microscopy identification of typical morphologic features may not be sufficiently sensitive [28].
To confirm the diagnosis of chancroid, laboratory tests can also be used, such as Gram staining of ulcer swabs which is not definite but can be used in conjunction with culture and PCR. Although these tests are not commonly available in many centers, a definitive diagnosis of chancroid is based on the isolation of Haemophilus ducreyi on a particular medium (chocolate agar) [1].
The practice of aspirating pus from the bubo or scraping from the ulcer's base. Vancomycin hydrochloride-enriched chocolate agar is one type of culture medium [29]. One way to change these methods is to increase sensitivity by using multiple media. Note that since H. ducreyi requires X-factor, or haematin, the media must be enriched with blood that has been carefully heated to liberate the growth factor haematin from hemoglobin. Subsequently, the infected plate needs to be incubated in an atmosphere with 3-5% CO2 and a high relative humidity of roughly 100%. This can be made with a candle jar full of wet plates. The plates should then be incubated at a lower temperature of 33-35°C.
The colonies of H. ducreyi after being incubated for 24 to 48 hours, appear waxy when scraped up with a wire loop. Sensitivity testing on Mueller-Hinton agar is carried out for isolates after the colonies have been sub cultured. Preserving cultures for future study is the better option. This can be done by freezing defibrinated rabbit blood at -70°C or lyophilizing in serum-inositol for long-term preservation [29].
In Gram staining, H. ducreyi is a Gram-negative rod and also a catalase negative organism. H. ducreyi reacts positively to Nitrate reductase, alkaline phosphatase but weakly reactive to oxidase and negatively to prophyrin. The bacterium is a non-glucose, maltose, xylose, sucrose and arabinose fermenter. Gram staining alone is not definitive, it should be used in conjunction with culture and polymerase chain reaction.
Detection of nucleic acid DNA by amplification techniques such as polymerase chain reaction (PCR) using nested techniques. Expert opinion has estimated that in endemic areas, a positive H. ducreyi culture is achievable in 60-80 percent of patients considered to have chancroid on clinical grounds [30]. The nucleic acid amplification test (NAAT) is a multiple PCR assay with a high detection rate; however no molecular assays have been approved by the Food and Drug Administration (FDA) for the diagnosis of chancroid. Other diagnostic tests include testing for other common STIs like; syphilis, HSV, gonorrhoea, chlamydia, and HIV. It is critical to distinguish chancroid from other genital ulcers such as syphilis, herpes, and granuloma inguinale, as their treatment and management varies, [31].
Given the poly-microbial flora of many ulcers, microscopy is only 50% sensitive compared to culture and prone to multiple errors. The most sensitive method is PCR, which has been shown to be 95% more sensitive than culture: On the other hand, compared to PCR, culture may only be 75% sensitive. However, Taq polymerase inhibitors present in DNA preparations extracted from genital ulcer specimens may cause PCR to be negative in several cultures-proven chancroid cases. 9. For the simultaneous amplification of DNA targets from HSV types 1 and 2, H. ducreyi, and Treponena pallidum, a multiple PCR assay has also been developed; however, it is only commercially available for research purposes [30].
Serology: Many epidemiological studies have found that serologic diagnosis of chancroid is helpful when employing enzyme-linked immunoassays (EIAs) with lysed whole cells, lipo-oligosaccharide (LOS), or outer membrane proteins (OMPs) as the antigen source [11,12]. But for a given patient, the approach is insensitive, specific, and unable to discriminate between a recent versus a remote infection [30].
Since the management and treatment of chancroid ulcer and other vaginal ulcers differ, it is important to make this distinction. Syphilis, granuloma inguinale, and herpes simplex virus (HSV) are other common sexually transmitted infections (STIs) that can result in genital ulcers [7]. Differentiating between these diseases can be aided by the clinical manifestation and appearance of the ulcers. For instance, HSV ulcers are usually painful and have a characteristic cluster of tiny vesicles, but syphilis ulcers are usually painless and have a hard base [7].
(Figure 5)
It is possible to avoid chancroid ulcers by taking the following precautions. Safe Sexual Behavior: The major way that chancroid is spread is through sexual contact. Thus, to lower the risk of chancroid transmission/infection, refrain from having sex or practice safe sex, such as consistently and correctly using latex condoms during vaginal, anal, and oral sex [32]. Reducing the number of sexual partners can assist in lowering the chance of developing chancroid. Quick Diagnosis and Intervention.
It is critical to get tested if chancroid is suspected and to start treatment as soon as a diagnosis is made. To guarantee that the infection is cured, take the antibiotics for the entire recommended duration as directed by a medical professional. Effective antimicrobial therapy stops the infection from spreading to other people in addition to curing the infection and treating its clinical symptoms. Without treatment, chancroid can persist longer and may lead to complications. Refusing to have Intercourse: Refrain from having intercourse until the infection has fully healed and the antibiotic treatment has been completed.
The following actions are also necessary for the management of chancroid in addition to the previously listed ones: Notifying and Treating Partners: When a person is diagnosed with chancroid, they should also test their partners for the infection. In order to stop the spread of the illness, they should also receive treatment if they are diagnosed. Monitoring and Reporting: Health officials must keep an eye on the prevalence of chancroid and notify the relevant public health agencies of any cases they come across. This makes it easier to spot disease outbreaks and trends in incidence. Education and Public Awareness: A reduction in the disease's incidence can be achieved by teaching people about the dangers of chancroid and how to avoid it. Promoting safe sexual behaviors such as condom use, limiting the number of sexual partners, and seeking immediate medical attention in the event of symptoms are all part of this.
Antimicrobial Resistance Monitoring: To make sure that treatment is still effective, it is critical to keep an eye on the bacteria that cause chancroid's susceptibility to various antibiotics. This entails determining the bacteria's antibiotic susceptibility and tracking how resistance patterns evolve over time [32]. The antibiotics of choice include the following: Azithromycin 1g oral single dose, Ceftriazone 250mg which is taken intramuscularly and as a single dose, Erythromycin 500mg oral treatment 3 times a day for 7 days. Resistance to certain antibiotics has been reported, necessitating susceptibility testing [33]. Treatment should be guided by local resistance patterns and alternatives may be needed in some areas [32].
In general, bacterial infection called chancriod ulcer causes painful genital ulcers and enlargement of the lymph nodes in the groin area. It is spread through sexual contact. Appropriate antibiotics can easily treat it, though longer medication regimens are necessary for HIV patients because of slower healing processes. Safe sexual practices, such as limiting the number of sexual partners, using condoms correctly and consistently, and seeking early testing and treatment, are recommended in order to prevent chancroid. Essential elements of chancroid management include partner notification, surveillance and reporting, education and awareness, and monitoring of antibiotic resistance. It is critical to finish the entire antibiotic course and show up for follow-up appointments to ensure the infection has been cured. Ulcers heal in two weeks, and if necessary, painkillers can be taken.
Copyright: © 2024 Ikpeama Roseanne Adah., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.