Sara Mohammed Alsaigh, Reem Hamoud Alrashoudi and Ayesha Mateen*
Clinical Laboratory Sciences Department, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia
*Corresponding Author:Ayesha Mateen, Clinical Laboratory Sciences Department, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia
Received: August 21, 2024; Published: September 06, 2024
Citation: Ayesha Mateen., et al. “In-Silico Analysis of Mn₃O₄ Nanoparticles Interaction with GDP-Mannose 6-Dehydrogenase: Potential for Inhibiting Biofilm Formation in Pseudomonas aeruginosa". Acta Scientific Microbiology 7.10 (2024):04-08.
The algD gene encodes GDP-mannose dehydrogenase, a key enzyme involved in the biosynthesis of alginate, a major exopolysaccharide produced by P. aeruginosa and other bacteria.
The aim of the study is to analyze molecular interaction of Mn3O4NPs and GDP-mannose dehydrogenase using In-silico approaches.
The molecular docking studies were conducted using Autodock 4.2 software and the protein structures were retrieved from UniProtKB servers.
In-silico molecular docking, analysis reveals insights into the binding efficacy of Mn3O4NPs with GDP-mannose 6-dehydrogenase protein exhibited highest binding energy (-6.42 kcal/mol), suggesting a potent inhibitory effect on biofilm formation.
In conclusion, Mn3O4NPs were found to possess the potential to disrupt biofilm integrity by interaction with GDP-mannose 6-dehydrogenase biofilm-associated protein.
Keywords: GDP-Mannose Dehydrogenase; Mn3O4NPs; Autodock 4.2 Software and algD Gene
Mn3O4NPs: Manganese Oxide Nanoparticles; RMSD: Root Mean Square Deviation; KI: Inhibition Constant; THR: Threonine; CYS: Cysteine; TRP: Tryptophan; HIS: Histidine; VAL: Valine; TYR: Tryptophan
Biofilms are complex, surface-attached microbial communities encased in a self-produced extracellular matrix, which provides structural stability and protection to the constituent cells [1]. These biofilms are of particular interest due to their role in persistent infections and resistance to antimicrobial treatments [2]. One of the key components of the biofilm matrix in many bacteria, including Pseudomonas aeruginosa (P. aeruginosa), is alginate, a high-molecular-weight polysaccharide that significantly contributes to the physical and functional characteristics of biofilms [3].
The alginate produced due to the action of the algD gene enhances the structural integrity of biofilms, making them more robust and difficult to disrupt. This is particularly important in chronic infections, where P. aeruginosa biofilms are known to be highly resistant to treatment [4,5].
The gene algD encodes GDP-mannose dehydrogenase protein that catalyzes the production of GDP-mannuronic acid from GDP-mannose and is particularly vital for producing alginic acid, which creates a gel-like substance necessary for biofilm maturation and stability [6,7].
In recent years, nanotechnology has emerged as a promising avenue for developing innovative strategies to combat bacterial biofilm formation [8]. Manganese oxide nanoparticles (Mn3O4NPs) have unique properties and are promising in preventing bacterial biofilm formation. Previous studies have shown that chemically synthesized Mn3O4-MnO2 nanocomposites are effective against P. aeruginosa bacteria. Mn3O4NPs have potential antibacterial effects, but more research is needed to understand their impact on biofilm formation at the genetic and molecular levels [9-11].
The present study has been conducted to analyze In-silico molecular interaction of Mn3O4NPs and GDP-mannose dehydrogenase protein binding efficacy which encodes biofilm formation genes. This research study may provide valuable information for developing personalized and environmentally friendly nanotherapeutics. We aim to contribute innovative biofilm control strategies in clinical and environmental settings.
Molecular docking studies were conducted to determine the binding mode and interactions between Mn3O4NPs and the protein as GDP-mannose 6-dehydrogenase protein (algD gene) (ID:P11759), belongs to P. aeruginosa, using Autodock 4.2 software [12]. The protein structures were obtained from UniProtKB servers [13].
The Molecular docking was performed using the protein structure of biofilm-formation genes of P. aeruginosa as receptors and Mn3O4NPs as ligands. The search grid of the GDP-mannose 6-dehydrogenase protein was identified as coordinates of central grid Point_x = -1.885, center_y = -0.038, and at a spacing of 0.375 Å. Coordinates of the central grid point of the pelF protein were identified as center x = -1.781, center y = -1.365, and center z = 2.903 at a spacing of 0.375 Å. Coordinates of the central grid point of the pslD protein were identified as center_x = 2.666, center_y = 0.294, and center z = -4.200 at a spacing of 0.375 Å. The analog Mn3O4NPs have been retrieved from the chemspider website [14].
All other parameters were set to the default values for the Auto Dock software program and are not mentioned herein. The compound with the least binding affinity value was the best-scoring compound. Discovery Studio Visualizer v24.1.0.23298 software and PyMOL Molecular Graphic System v2.5.8 [15] were used to analyze all results visually.
The interaction between GDP-mannose 6-dehydrogenase protein and Mn₃O₄NPs (Figure 1). was characterized using molecular docking techniques. The binding energy of the interaction was calculated to be -6.42 kcal/mol. This negative value indicates a thermodynamically favorable interaction between GDP-mannose 6-dehydrogenase and Mn₃O₄ NPs. The root mean square deviation (RMSD) of the protein upon binding to Mn₃O₄ NPs was found to be 7.14 Å. This significant RMSD value implies substantial conformational changes in the protein structure. The inhibition constant (KI) was determined to be 0.51 mM, indicating that Mn₃O₄ NPs are potent inhibitors of GDP-mannose dehydrogenase. A total of 11 hydrogen bonds were identified between GDP-mannose 6-dehydrogenase and Mn₃O₄ NPs, with bond distances ranging from 2.591 to 3.437 Å. The hydrogen bonds involved the following amino acid residues: THR212, CYS213, TRP216, HIS217, VAL215, and TYR211. These residues are located near the enzyme's active site, indicating that Mn₃O₄ NPs may interact with and potentially disrupt the enzyme's catalytic function (Table 1) (Figure 2).
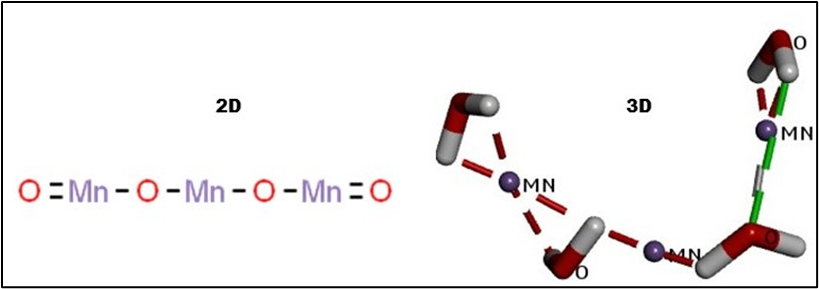
Figure 1: Structure of Mn3O4 NPs.
![Figure 2: The Molecular docking 2D and 3D images of Biofilm-formation proteins (receptor) with Mn3O4 NPs (Ligand).
[Figures 1a, 1b, and 1c represent the molecular docking complex of GDP-mannose 6-dehydrogenase protein (algD gene) (Receptor) and Mn3O4 NPs (Ligand), 1b represents the 3D structure and 1c represents the 2D structure].](https://actascientific.com/ASMI/images/ASMI-07-1430_figure2.png)
Figure 2: The Molecular docking 2D and 3D images of Biofilm-formation proteins (receptor) with Mn3O4 NPs (Ligand).
[Figures 1a, 1b, and 1c represent the molecular docking complex of GDP-mannose 6-dehydrogenase protein (algD gene) (Receptor) and Mn3O4 NPs (Ligand), 1b represents the 3D structure and 1c represents the 2D structure].
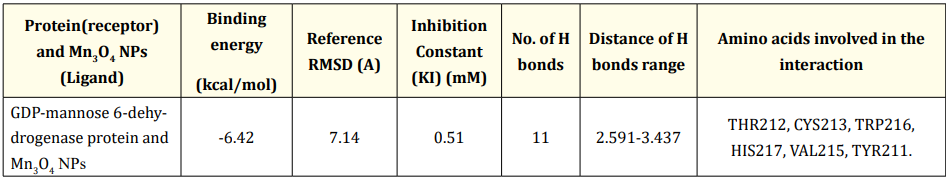
Table 1: Molecular docking analysis of Biofilm-formation proteins and Mn3
O4 NPs.
GDP-mannose 6-dehydrogenase protein (algD gene); pelF protein (pelF gene); pslD protein (pslD gene).
The In-silico molecular docking study of Mn3O4NPs with GDP-mannose 6-dehydrogenase biofilm-formation protein in P. aeruginosa reveals insights into Mn3O4NPs potential as biofilm formation inhibitors.
The interaction between GDP-mannose 6-dehydrogenase, a key enzyme in alginate biosynthesis encoded by the algD gene, and Mn3O4NPs was investigated to assess the potential impact on the enzyme's function and, consequently, on biofilm formation in P. aeruginosa [16]. The binding energy of -6.42 kcal/mol indicates a relatively strong and favorable interaction between GDP-mannose dehydrogenase and Mn₃O₄NPs. This suggests that the nanoparticles have a significant affinity for the enzyme, which could potentially interfere with its activity [17].
The root mean square deviation (RMSD) value of 7.14 Å suggests substantial conformational changes in the protein upon binding to Mn₃O₄ NPs. Such a high RMSD indicates that the binding of nanoparticles induces significant structural alterations in the enzyme, which could affect its stability, folding, and catalytic efficiency. This level of structural deviation suggests that the Mn₃O₄ NPs might disrupt the enzyme's normal function by altering its three-dimensional structure, particularly around the active site [18].
The inhibition constant (KI) of 0.51 mM further supports the notion that Mn₃O₄NPs are potent inhibitors of GDP-mannose dehydrogenase. The low KI value indicates a strong inhibitory effect, implying that even at low concentrations, Mn₃O₄ NPs can significantly reduce the enzyme's activity. Given the enzyme's critical role in alginate biosynthesis, such inhibition could lead to a decrease in alginate production, thereby affecting the formation and maintenance of biofilms, which are essential for Pseudomonas aeruginosa survival and pathogenicity.
The interaction analysis revealed the formation of 11 hydrogen bonds between GDP-mannose dehydrogenase and Mn₃O₄ NPs, with bond distances ranging from 2.591 to 3.437 Å. These hydrogen bonds involve key amino acid residues—THR212, CYS213, TRP216, HIS217, VAL215, and TYR211—that are located near the enzyme's active site. The involvement of these residues suggests that Mn₃O₄ NPs may interfere with the enzyme's catalytic activity by disrupting essential interactions within the active site, potentially leading to a reduction in substrate binding or catalytic efficiency.
Overall, the data suggest that Mn₃O₄NPs have a significant impact on the structure and function of GDP-mannose dehydrogenase. The strong binding affinity, coupled with the substantial conformational changes and potent inhibitory effect, indicates that these nanoparticles could effectively inhibit the enzyme's activity. This inhibition could result in reduced alginate production, thereby impairing biofilm formation in P. aeruginosa. Considering the critical role of biofilms in bacterial resistance to antibiotics and host immune responses [19]. Mn₃O₄NPs could be explored as a potential therapeutic agent for controlling biofilm-associated infections [20,21].
Further studies, particularly in vivo experiments, are needed to confirm these findings and to explore the therapeutic potential of Mn₃O₄NPs. These investigations would provide valuable insights into the feasibility of using nanoparticles to target key enzymes in biofilm formation, offering a novel approach to combat persistent bacterial infections.
To conclude, Mn₃O₄NPs exhibited as valuable inhibitor of biofilm formation in P. aeruginosa by targeting the GDP-mannose 6-dehydrogenase enzyme. As the bacterial biofilms plays a critical role in antibiotics resistance, Mn₃O₄ NPs could be useful as a novel therapeutic agent against biofilm-associated infections. However, further in vivo studies are necessary to confirm their efficacy and safety, paving the way for new treatments against persistent bacterial infections.
No financial support was received for this research.
The authors have no conflict of interest.
Conception and design of the study was carried out by Sara Mohammed Alsaigh acquisition of data, analyses, and interpretation was Ayesha Mateen and critical revision of the manuscript was done Reem Hamoud Alrashoud.
The authors were thankful to the department of Clinical Laboratory science, King Saud University, for encouraging to conduct the present research work.
Copyright: © 2024 Ayesha Mateen., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.