Aakhila A* and Feminamol V M
Dissertation Under the Guidance of Ms. Neethu George, Assistant Professor, Department of Microbiology, Presentation college of Applied Sciences Puthenvelikkara, Ernakulam, Kerala, India
*Corresponding Author: Aakhila A, Dissertation Under the Guidance of Ms. Neethu George, Assistant Professor, Department of Microbiology, Presentation college of Applied Sciences Puthenvelikkara, Ernakulam, Kerala, India.
Received: February 18, 2021; Published: March 29, 2021
Breast milk is a nourishment and medicinal shield for the new born. Its various benefits include strengthening baby’s immune system, anti-inflammatory functions, lowering the risk of allergies, mental ailments etc.
In this study the antimicrobial activity of breast milk against some neonatal pathogens (Staphylococcus aureus and Candida albicans) were evaluated. Antibacterial activities of different antibiotics were tested using Kirby Bauer disc diffusion method. Breast milk from different mothers as well as cow milk were subjected to antimicrobial assay by agar well diffusion. Result showed that the breast milk has antibacterial activity against the tested organisms compared to cow milk which have no antibacterial effect.
Keywords: Breast Milk; Neonatal Pathogens; Antibacterial Effect
Human milk contains many of biologically active components that contribute to the short- and long-term benefits of breast feeding. Breast milk is a nourishment and medicinal shield for the new born. Its various benefits include strengthening baby’s immune system, lowering risk of allergies, chronic diseases, mental ailments etc. [16]. Human milk contains several factors that serve to shield the infants against numerous bacteria, viruses, and fungi [20]. Its antibacterial effect due to the presence of antimicrobial peptides (AMP) like lactoferrin, alpha defensins, beta defensin, lysozyme, lacto-peroxidase [8].
Breast milk contains higher levels of AMP than those present in formula feed or cow milk. All these factors in breast milk lower the occurrence of number of bacterial infections in babies such as ear infection, respiratory illnesses, and diarrhea [30]. Lactose in breast milk is a source of energy for the infant. Other than lipids and lactose, human milk oligosaccharides (HMOs) are the third most abundant components of human breast milk.
HMOs are diverse, conjugated short chain and long chain glycans 93-15 monosaccharide units found at concentrations of 20-25 g/L in the colostrum, the first milk, to 5-20 g/L in the mature milk. The diversity, complexity and concentration of HMOs are unique to humans, and cannot be provided by milk of domestic animals such as cows, sheep or goats. The concentration and composition of HMOs is individual and varies during the period of lactation, complimenting the needs of infant [22]. In addition to their prebiotic effects on beneficial bacteria, HMOs also directly deter colonization by pathogens (not only bacteria but also virus) by acting as anti-adhesive antimicrobials. The protective mechanism of HMO has been reported for several diarrhoea causing pathogens such as Campylobacter jejuni and Salmonella fyris, but also against pathogenic Escherichia coli, Pseudomonas aeruginosa and Streptococcus pneumoniae. HMOs even show antimicrobial activity against pathogenic Staphylococcus aureus and Acinetobacter baumannii, the major source of nosocomial infections [1]. Neonatal sepsis is a significant cause of morbidity and mortality among neonates worldwide [31]. It may occur through transplacental infection [17]. Neonatal sepsis is categorized into early and late onset sepsis. Early onset neonatal sepsis (EONS) occurs within 72, while the late-onset neonatal sepsis (LONS) occurs between 72 h to 90 days [29]. The bacterial agents involved in early-onset sepsis include group B Streptococcus (GBS), Escherichia coli, coagulase negative Staphylococcus, Haemophilus influenzae and Listeria monocytogenes [33]. The organisms involved in the late-onset sepsis include coagulase negative staphylococci (CONS), Staphylococcus aureus, Klebsiella pneumoniae, Escherichia coli etc. [12].
Standard treatment of neonatal sepsis includes the use of antimicrobial agents. Antibiotics are continued, changed, or discontinued depending on the laboratory test results; extend of clinical suspicion and cultures [21]. Use of appropriate antibiotics according to bacterial profile and culture sensitivity results would minimize the risk of severe morbidity and mortality and help in reducing the emergence of multidrug–resistant organisms [6].
The present study was carried out to determine the antibacterial effect of human breast milk against common neonatal pathogens and common isolates of neonatal sepsis.
Breast milk is considered to be the best source of infant growth and nutrition. Evidences convey that breast milk contains a variety of bioactive agents that improve the function of the gastrointestinal tract, the immune system, brain development. Thus, breast milk is necessary for optimal infant growth and development. Recently, studies have further suggested that breast milk reduces infant late metabolic diseases, protecting against obesity and type 2 diabetes [27]. Fatty acids present in breast milk has antimicrobial function in the intestinal tract. Interestingly, fatty acids found in algae also have antimicrobial activity against both gram-positive and gram-negative bacteria [10]. The intrinsic components of milk have local antipathogenic effects that supplement the infant’s innate immunity. This includes substances such as free fatty acids (FFA), monoglycerides, antimicrobial peptides (AMP), which bind diarrheal pathogens [26].
In addition to these, there are other factors present in the breast milk support infant’s immune system including bifidus factor, lysozyme, lactoperoxidase, lactoferrin, lipoprotein, lipase. epidermal growth factor in breast milk may stimulate the maturation of the gastrointestinal epithelium as a barrier. There is convincing evidence that breast feeding may reduce the incidence of gastrointestinal infections [19]. In a study, the protective effect of breast feeding against enterovirus infections was primarily mediated by maternal antibodies in breast milk [24]. In addition, infants who were breast fed for >4 month showed a three times reduced incidence of severe respiratory tract infection, as compared with infants who were not breast fed [3]. Breast milk also provides protection against otitis media and urinary tract infections [15].
Neonatal sepsis is classified into “early onset” if it occurs within the first week and as “late onset” if occurring after the first week until the end of the neonatal period [14]. The findings of this review suggest that Klebsiella spp, E. coli, and S. aureus are major causes of infections the first week of life. However, data for the first week of life from community settings are particularly scarce, and the contribution of environmental factors in the causation of infection among hospital born babies cannot be excluded. Beyond the first week of life—although based on relatively few reports of community-acquired infections—E. coli, GBS, and S. aureus, as well as S. pneumoniae, S. pyogenes, and Salmonella spp are commonly isolated [32].
A similar preponderance of gram-negative organisms and S. aureus is also noted in home-delivered babies, although data again were extremely limited. From the WHO-sponsored YIS study from Philippines, the authors [7] also note a predominance of gram-negatives among home-delivered babies (76% of 21 babies), although the etiological break up was not presented.
Although GBS–relative to other organisms and relative to data from industrialized countries [23] – were not as frequently reported, particularly in the first week of life, they appear common in African countries and very uncommon in the South Asian region. However, intercountry variations are also apparent, with some South Asian reports suggesting higher prevalence of GBS and some African reports the converse, or even no GBS isolates [13]. The reasons for such inter-regional variations are not clearly understood, and variations in risk factors such as vaginal colonization, strain virulence, antibody levels, or cultural practices are thought to contribute [2].
Among pathogens in the 7 to 59-day period, only a limited number of facility-based studies clearly attempted to exclude hospital-acquired infections. These data were dominated by a single Malawian study [18], and more than half of remaining data were from multicenter Young Infant Studies (YIS) and rural Kenya [4]. Data from YIS indicated that S. pneumoniae, S. aureus, E. coli, S. pyogenes, and nontyphoidal Salmonella species were important pathogens beyond the first week of life, whereas in Kenya and Malawi, in addition to above pathogens, GBS were also common. The spectrum in the late onset age group can, in general, be considered a transitional phase between neonatal and post neonatal periods–where organisms associated with neonatal sepsis (S. aureus, E. coli, GBS), as well as those associated with post neonatal sepsis (S. pyogenes, S. pneumoniae), are equally important. It is worth noting that published information on community-acquired sepsis in the late neonatal and post neonatal period from South Asia is strikingly absent, although this region contributes very heavily to the global burden of neonatal and child mortality.
Staphylococcus aureus is a facultative anaerobic Gram-positive bacterium whose incidence ranges from skin, soft tissue, endovascular to wound infections. Candida albicans is an opportunistic fungus that can cause superficial, localized, and systemic infection. Gram-positive pathogens played a major role in etiology of sepsis in the post neonatal and early infant period and S. pneumoniae emerged as the single most important pathogen in this age group. Other important pathogens in this group included Salmonella species and Haemophilus influenzae. H. influenzae are almost certainly under-estimated, especially in South Asia because of inadequate bacteriological laboratory resources to isolate this fastidious pathogen, and frequent use of antibiotics by children before submitting specimens for culture. Indeed, S. pneumoniae, H. Influenzae, and Salmonella are frequently reported in etiology of pneumonia, meningitis, and septicemia in children under 5 years of age from African and South Asian countries, and from other regions, especially when nonculture methods such as antigen detection are used [25]. However, the post neonatal and early infant groups are often excluded or not disaggregated from infant age groups. All of these factors contribute to the paucity of data available in this age group category. Both gram-positive and gram-negative isolates showed a high resistance to cephalosporins, penicillin, and amoxyclav in the current study, it was observed that antibiotic resistance among the gram-positive isolates was highest to penicillin (87%) followed by amoxyclav (66%). Similar reports of high resistance to ampicillin (71%) were reported by [5]. All the gram-positive isolates were sensitive to vancomycin similar to a study by [33] in the present study, 41% S. aureus isolates were found to be methicillin-resistant, compared to 11.1% reported by [12]. Gram-negative isolates showed a high resistance to all cephalosporins which is similar to the resistance pattern reported by Klebsiella pneumoniae. showed resistance to all antibiotics tested except imipenem. In our study, the 48% were ESBL producers as compared to 72% as reported by [5].
Neonatal septicemia is a life-threatening situation therefore rapid treatment with antibiotics is essential. Treatment of neonatal sepsis mainly with amoxicillin and aminoglycoside. S. aureus and gram-negative isolates were found to be resistant to amoxicillin and aminoglycoside. So selection of antibiotic is the key step in the treatment. In the view of above the strategy of antibiotic usage in the hospital must be reviewed [28].
ObjectivesOral, pus and ear swabs from nearby locality and hospitals were taken. Swabs were pooled in nutrient broth tubes.
Isolation of neonatal pathogens Materials requiredClinical specimens were streaked on nutrient agar plates and incubated at 37˚C for 24 hours. Colonies so obtained on nutrient agar plates were further inoculated into peptone water and identified on the basis of:
Strains were then stored as slant at 4˚C till use.
Biochemical reactionsCatalase test
Coagulase test
Germ tube test
Antimicrobial susceptibility test (Kirby Bauer disc diffusion method).
Antimicrobial susceptibilities of isolated pathogens to the selected antimicrobial agents were determined (CLSI).
Materials requiredThe present study was aimed at evaluating the anti-bacterial properties of human breast milk against pathogens that are commonly associated with infections of new-born and infants. This was done by collecting different clinical specimens from hospitals in the locality. A total of 4 strains isolated from 3 different samples were designated as S1 S2 S3 and F1based on their colony (Table 1-7, Plate 1-12).
S1, S2, S3 were identified as Staphylococcus aureus and F1 were identified as Candida albicans respectively.
| Clinical samples | Isolates |
|---|---|
Ear Swab |
S1 |
Pus swab 1 |
S2 |
Pus swab 2 |
S3 |
Oral swab |
F1 |
Table 1: Different morphology isolates from clinical specimen.
| Isolate | Colony morphology |
|---|---|
S1 |
Small, pin head size, golden yellow, convex colonies |
S2 |
Small, pin head size, yellow, raised, convex colonies |
S3 |
Small, pin head size, creamy yellow, convex colonies |
Table 2: Bacterial colony characteristics.
| Isolate | Colony morphology |
|---|---|
F1 |
Large smooth white to cream colony |
Table 3: Fungal colony characteristics.
| S.no | Isolates | Gram staining | Morphology |
|---|---|---|---|
1. |
S1 |
+ |
Cocci in pairs and chains |
2. |
S2 |
+ |
Cocci in pairs and clusters |
3. |
S3 |
+ |
Cocci in clusters |
Table 4: Different morphology isolates from clinical specimen.
| S.no | Isolates | Gram staining | Morphology | Germ tube |
|---|---|---|---|---|
1. |
F1 |
+ |
Oval budding cells |
+ |
Table 5: Morphological characterizations of fungal isolate.
+: Positive
-: Negative.
| S.no | Isolates | Catalase | Coagulase | Mannitol salt agar |
|---|---|---|---|---|
1. |
S1 |
+ |
+ |
+ |
2. |
S2 |
+ |
+ |
+ |
3. |
S3 |
+ |
+ |
+ |
Table 6: Biochemical characterizations of bacterial isolates.
+: Positive
-: Negative.
Morphological characters and biochemical reactions of the isolates were studied and finally characterized and identified by following Bergey’s Manual of Determinative Bacteriology.
Gram staining reactions of isolates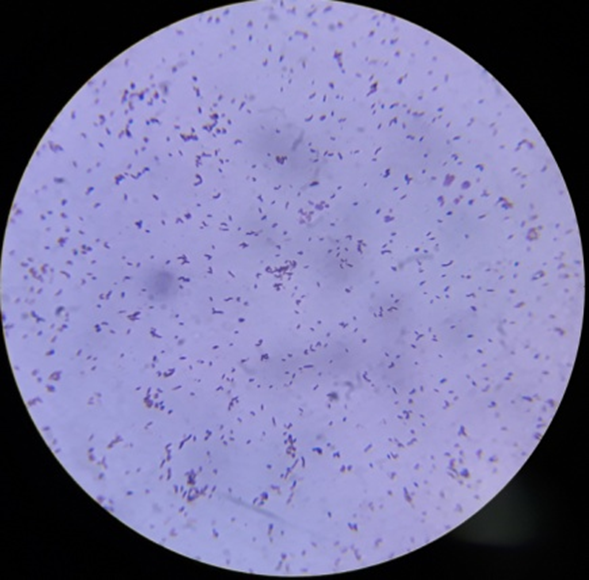
Plate 1: S1.
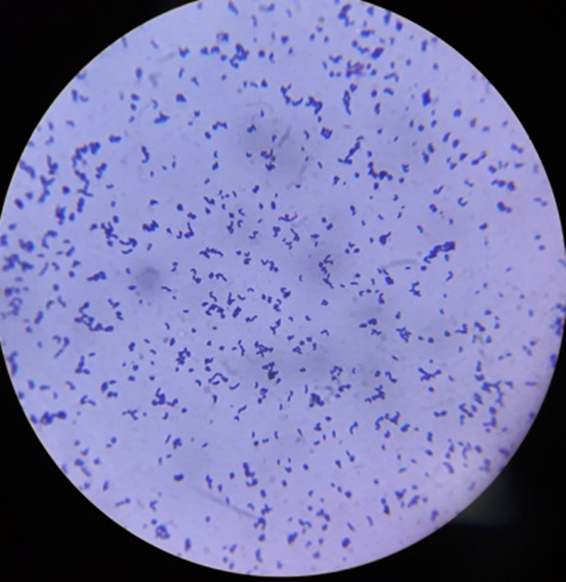
Plate 2: S2.
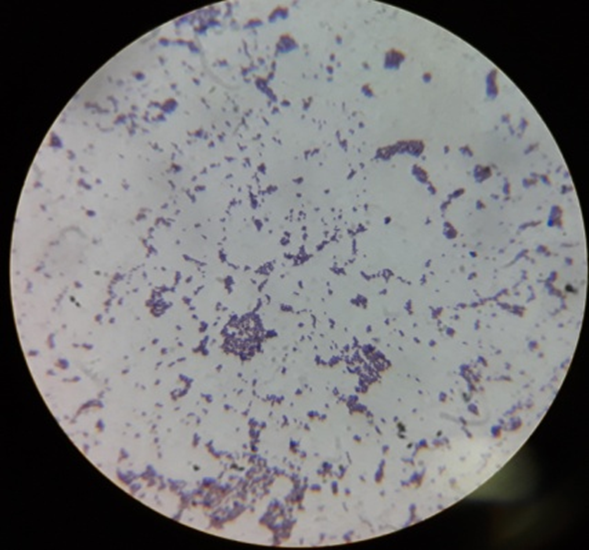
Plate 3: S3.
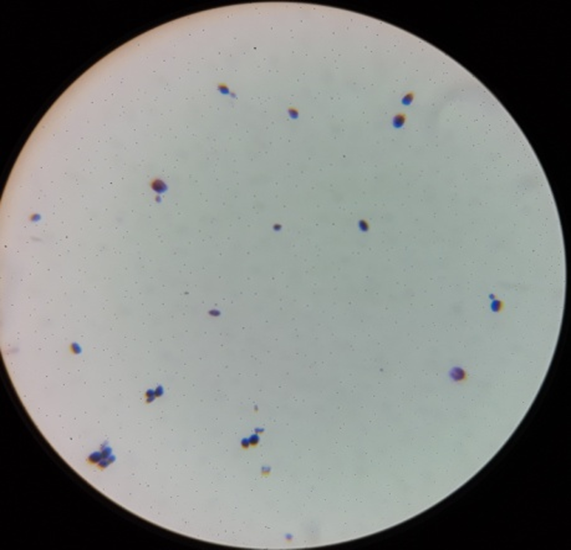
Plate 4: F1
| S.no | Isolates | Genera |
|---|---|---|
1. |
S1 |
Staphylococcus aureus |
2. |
S2 |
Staphylococcus aureus |
3. |
S3 |
Staphylococcus aureus |
4. |
F1 |
Candida albicans |
Table 7: Identified bacterial and fungal isolates.
Plate 5: F1.
The results also showed that the numerical density of lice was higher than that of fleas on the body of a gray-bellied rat. Regardless of months, lice exhibited the highest population density, whereas, fleas showed the least population density, this may be attributed to that fleas visit rats for feeding only, but lice are permanent parasites on hosts. The results similar with Embarak [14]; Kia., et al. [5] and Desoky., et al. [15].
Bacterial and fungal isolates on suitable media on nutrient AGAR and SDA
Plate 6: S1.
Plate 7: S2.
Plate 8: S3.
Plate 9: F1.
Plate 10: S1.
Plate 11: S2.
Plate 12: S3.
All the bacterial strains isolated were tested for antibiotic susceptibility test using Kirby bauer method.
All the bacterial isolates were sensitive to both Gentamicin and Rifampicin. However, all the isolates were resistant to Penicillin (Table 8, Plate 13-15).
| Isolate | Penicillin | Gentamicin | Rifampicin | Amoxicillin |
|---|---|---|---|---|
S1 |
R |
20 mm (S) |
26 mm (S) |
30 mm (S) |
S2 |
R |
19 mm (S) |
30 mm (S) |
R |
S3 |
R |
19 mm (S) |
16 mm (S) |
R |
Table 8: Antibiotic susceptibility tests of the bacterial isolates.
R: Resistant; S: Sensitive.
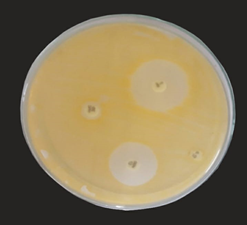
Plate 13: S1.
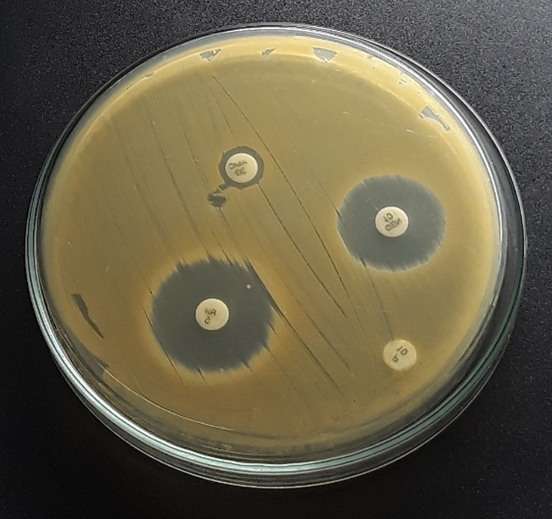
Plate 14: S2.
Plate 15: S3.
The antibacterial efficacy of breast milk and cow milk was evaluated using the agar well diffusion assay as per the method of CLSI.
Antibacterial activity of breast milkAntibacterial activity on isolate S1
Plate 16
Antibacterial activity on isolate S2
Plate 17
Antibacterial activity on isolate S3
Plate 18
Antibacterial activity on isolate F1
Plate 19
Human breast milk was evaluated for its anti-bacterial effect using agar diffusion assay against three strains of Staphylococcus aureus and Candida albicans. Human breast milk was far more superior in its anti-bacterial effect seen (in terms of zone diameter) than cow milk against all the four strains tested. Breast milk showed largest zone diameter for S.aureus S1 and S2 respectively. Cow milk showed insignificant activity against all the test strains.
In general, there are two mechanisms to explain the inhibitory effect of human breast milk. One is the interaction between specific constitute in milk with epithelial surfaces or with specific substances in the gastrointestinal lumen during digestion of milk while the second mechanism is the possible modulation of infant’s immune system by protective factors in the milk which results in selective production of immune factors in the infant [9]. The intrinsic components of milk or partially digested products of human milk, which have local antipathogenic effects that supplement the infant’s innate immunity. This includes substances that function as prebiotics such as free fatty acids (FFA), monoglycerides, antimicrobial peptides (AMP), which bind diarrheal pathogens [26].
First and foremost, I bow in profound gratitude to GOD ALMIGHTY for his graceful blessings showered upon us for the successful completion of this work and who has been my strength throughout the course of this project work. Before I proceed, I would like to add a few heart full words for people who were part of this endeavor in numerous ways and people who gave great support right from the beginning.
I express my deep sense of gratitude to our Rev. Fr. Shaijan M Kalathil, Manager, Presentation College of Applied Sciences, Puthenvelikkara, Ernakulam to allowing me to do this project.
I express my thanks to Mr. Feby FAS, Principal, Presentation College of Applied Sciences Ernakulam for his moral support.
I am ever grateful to Dr. Rashmi P.A (Head of the Department of Microbiology) and Ms.Dayana Joseph (class in charge) and those who supported me during my project work.
It’s my privilege to express my sincere gratitude to my guide Ms. Neethu George, Assistant Professor, Department of Microbiology for her valuable help guidance and encouragement rendered to me throughout my project work.
I am thankful to all the teaching and non-teaching staff including lab assistants of the Department of Microbiology for their help, assistance and co-operation.
I thank my friends for their valuable support and help.
Last but not least my heart full thanks to my family whose love, prayers, moral support and encouragement lifted me every time I encountered possibilities.
Citation: Aakhila A and Feminamol V M. “Antimicrobial Activity of Breast Milk Against Neonatal Pathogens". Acta Scientific Microbiology 4.4 (2021): 197-207.
Copyright: © 2021 Aakhila A and Feminamol V M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.