Juan Quintero-Soto1, Fabián Espitia-Almeida1,2, Jaison Torres-Pacheco1, María Fragozo-Ramos3, Rita Sierra-Merlano3, V Alberto Laguna-Torres4, Hernando Pinzón-Redondo5 and Doris Gómez-Camargo1,2*
1UNIMOL Research Group, Faculty of Medicine, University of Cartagena, Colombia
2Doctorate in Tropical Medicine, Faculty of Medicine, University of Cartagena, Colombia
3Department of Internal Medicine. University Cartagena, Colombia
4Tropical Medicine Institute, National University of San Marcos de Lima, Peru and Visiting Professor University of Cartagena
5Department of Pediatrics, University of Cartagena-Napoleón Franco Pareja Children's Hospital-Casa del Niño, Colombia
*Corresponding Author: Doris Gómez-Camargo, UNIMOL Research Group, Faculty of Medicine, University of Cartagena, Colombia.
Received: February 26, 2021; Published: March 18, 2021
Background: Severe acute respiratory illness (SARI) is the main cause of death of infectious origin worldwide, and influenza is of special interest due to the availability of vaccines and specific antiviral therapy.
Objective. This work includes 2 objectives: (1). Describe the epidemiological and clinical characteristics of hospitalized patients with severe acute respiratory infection caused by influenza and other respiratory viruses in Cartagena-Colombia. (2). Report the status of immunization and/or vaccination against seasonal influenza.
Materials and Methods: This study was conducted in two hospitals located in Cartagena-Colombia, the study population included children and adults admitted to the emergency room with an acute infection process associated with influenza. The identification of infectious agents was done by RT-qPCR and multiplex PCR (FilmArray).
Results: 278 patients with potential eligibility for the study were recruited, of which 175 (62.9%) met the inclusion criteria. The most frequently detected viruses were RSV, rhinovirus, and influenza, each with 47.1%, 33.6%, and 17.3% of the cases, respectively. 5.6% of the positive cases of influenza were admitted to the ICU and died. The presence of comorbid disease, use of antivirals during hospitalization, and current vaccination was significantly low. Vaccination status for seasonal influenza was most common in <5 years. Several co-infections occurred and some of them were bacterial, Rhinovirus was the most frequent co-infecting viral agent.
Conclusion: The active surveillance model is a valuable tool to investigate the impact of respiratory viral diseases in hospitalized patients, providing information that allows the implementation of preventive and control measures.
Keywords: Influenza; Surveillance Network; Epidemiology; Virus; Vaccine
Acute respiratory infections (ARI) are a large group of diseases that affect the upper and lower respiratory system. Higher ARFs are generally not life-threatening, on the contrary, low infections are the fourth leading cause of death in the world with approximately 2.8 million deaths annually [1-3]. ARIs can be of viral, bacterial or mixed origin. In low-income countries, they are the main cause of morbidity and mortality from infectious disease in children under 5 years of age, older adults, and subjects with compromised immune systems [1].
The World Health Organization (WHO) recommends greater attention to ARIs caused by influenza viruses and rhinoviruses, as they are the most frequent causative agents of ILI (Influenza-like Illness) [4].
In the last two decades it has been possible to identify other viral agents that cause ILI by molecular methods such as the multiplex polymerase chain reaction (PCR-multiplex) [5], standing out as the cause of ILI and due to its impact on human health. enteroviruses, human coronaviruses (229E, NL63, OC43, HKU1), respiratory syncytial virus, human metapneumovirus, parainfluenza virus, human bocavirus, adenovirus and recently the SARS, MERS and SARS-CoV-2 coronaviruses [4,6-9].
Influenza can evolve to SARI with increased mortality in high-risk individuals such as children <5 years, pregnant women, the elderly, and the immunosuppressed [10]. Influenza causes SARI in approximately 5 million people and is the direct cause of 650,000 deaths each year worldwide [11]. Influenza outbreaks also generate economic losses due to absenteeism from work and decrease the quality of life of patients and their families [12], becoming a global problem of Public Health. Colombia seeks to improve the identification of influenza and differentiate it from other causes of ILI, to understand its epidemiology and control outbreaks [9]. For 2019, 1,317 cases of SARI were reported in Colombia, 62% of these were caused by respiratory syncytial virus, 18% by Influenza AH1N1, 8% Parainfluenza, 6% Seasonal Influenza A, 3% Influenza B and 3% adenovirus. Being the pediatric population <5 years and adults> 40 years the most affected [9].
Cartagena does not have an efficient ILI search system and SARI cargo. A tertiary objective is to identify ILI-related health comorbidities using the disease code system in Colombia.
All procedures were performed in accordance with the protocols approved by the GIHSN and informed consents were obtained from each patient. A headquarters coordinating physician reviewed the medical records daily to identify eligible participants. The inclusion of patients to the study was based on the main diagnosis at the time of admission. Patients with clinical symptoms of Influenza Type Illness during the seven days prior to admission and who were hospitalized one or two nights prior to enrollment were included.
Study design and patient selectionA cross-sectional study with multistage sampling was carried out, it was designed to provide information on active surveillance against influenza and other respiratory viruses of hospitalized patients in Cartagena-Colombia. The study population included children and adults admitted to the emergency room or specialized inpatient units of two hospital centers; Hospital Universitario de Caribe (HUC) and Fundación Hospital Infantil Napoleón Franco Pareja (FHINFP) in the period between March 2019 and February 2020.
Criteria for inclusion and exclusion of patientsPatients of all ages who were admitted for any condition associated with influenza were considered eligible for the study as previously described. Inclusion criteria: (1). Hospital admission within 24 to 72 hours before recruitment, (2). Be a resident of Cartagena, (3). Have a history of ILI with a maximum of 7 days prior to inclusion in the study, to define cases of ILI the definition of the European Center for Disease Control and Prevention (CDC) was used; patient presenting with a combination of at least ONE of the following systemic symptoms Fever, headache or general malaise, and at least ONE of the following respiratory symptoms: Cough, sore throat or shortness of breath/shortness of breath. Exclusion Criteria: (1). Patient who did not give informed consent, (2). Previous hospitalization ≤30 days before recruitment, (3). Non-resident of Cartagena, (4). Being institutionalized (living in a nearby coexistence center: asylum, prison, nursing home, orphanage, etc.). The confined military will be considered institutionalized.
Sample collection and processingTwo combined swabs were obtained per patient, all participants regardless of age had a nasopharyngeal swab taken, in addition, at> 14 years and adults a pharyngeal swab was taken, while <14 years and children had a nasal swab [13]. The swabs were placed in universal medium for viral transport (HealthLink) and stored at –80ºC until their subsequent analysis in the Molecular Research Unit laboratory (UNIMOL) of the University of Cartagena. Additionally, clinical and demographic variables were collected.
Sample processing for the detection of Influenza and other respiratory virusesThe viral RNA was extracted using the PureLink Pro 96 Viral RNA⁄DNA kit (Invitrogen) following the manufacturer's protocol and it was stored at -80ºC until use. Subsequently, the samples were analyzed for influenza A and B viruses using TaqMan probes for real-time reverse transcription PCR (RT-qPCR) according to the CDC manual [14], using the TaqMan Fast Virus 1-Step system. Master Mix (Applied Biosystems), following the manufacturer's instructions. Positive samples for influenza A were determined for the H3 and H1N1 pdm09 subtypes. All PCR processes were carried out on an ABI 7500 Fast equipment (Applied Biosystems). The samples that yielded negative results for influenza A and B were processed by multiplex PCR FilmArray (BioFire), using the respiratory panel 2.0 (Biomérieux) with specificity for respiratory pathogens (17 viruses and 3 bacteria).
Ethical aspectsThe study was reviewed and approved by the Ethics Committee of the University of Cartagena, written informed consent was obtained, and consent of the responsible companion was requested for minors. The consents and the information collected are in the custody of the UNIMOL laboratory. The risk for patients was less than a minimum (article 11 of ethical aspects of research in humans, resolution 8430 of 1993).
Statistical analysisThe database was registered in Excel and analyzed in SPSS version 25. The categorical variables were analyzed as absolute and relative frequencies. The numerical variables were valued by the Kolmogorov–Smirnov statistic to determine their normality, when the numerical variable followed the assumption of normality it was represented by the mean and standard deviation, when it did not follow the normal distribution it was represented by the median and interquartile range. Statistical differences were evaluated using the two-tailed Fisher's exact test and the Chi-square test, p < 0.05.
During the study period, data were collected from 278 patients (197 in FHINFP and 81 in HUC), of which 175 (62.9%) were eligible for the final analysis because they met the selection criteria (Figure 1).

Figure 1: Flow chart that summarizes the process of inclusion of patients to the study.
The demographic and clinical characteristics of the patients included in the study are presented in table 1. The age expressed as median and interquartile range was 2 [46-0] years, the male sex was more predominant with 62.2% (n = 109), 60.6% (n = 106) of the patients recruited in FHINFP were <5 years old because this is a hospital center specialized in pediatric medical care and in HUC all the patients recruited were aged ≥18 years. The presence of chronic diseases or comorbidities associated with severe influenza outcomes was low in the general population with 42.3% (n = 74), for FHINFP the comorbidity was present in 25.4% (n = 33) of the patients, while the HUC it was characterized by a significantly higher frequency with 91.1% (n = 41), p < 0.05. Another important factor that affects the frequency of severe cases of influenza infections is the use of antiviral drugs and vaccination. In our study, the administration of antiviral therapy in the entire population was frequently low; 99.4% (n = 174) of the patients reported not receiving antiviral therapy during hospitalization, vaccination against seasonal influenza was absent in 76.6% (n = 134) of the subjects, and 6.9% (n = 12) reported not knowing your vaccination status. Regarding vaccination by age, only 24.5% (n = 26) of children <5 years included in the study were vaccinated.
| Characteristics | FHINF n = 130(%) | HUC n = 45(%) | Total n = 175(%) |
|---|---|---|---|
Sex |
|||
M |
83 (63.8) |
26 (57.8) |
109 (62.2) |
F |
47 (36.2) |
19 (42.2) |
66 (37.8) |
Age (median in years: RI) |
1 (3 – 0) |
66 (82 – 66) |
2 (46 – 0) |
Age group (years) |
|||
< 2 |
86 (66.2) |
0 |
86 (49.2) |
2 - 4 |
20 (15.4) |
0 |
20 (11.4) |
5 - 17 |
7 (5.4) |
0 |
7 (4.0) |
18 - 60 |
9 (6.9) |
18 (40.0) |
27 (15.4) |
> 60 |
8 (6.1) |
27 (60.0) |
35 (20.0) |
Fever > 38 °C |
39 (30) |
34 (75.5) |
73 (41.7) |
Exposure to smoking |
|||
Yes |
27 (20.8) |
26 (57.8) |
53 (30.3) |
No |
103 (79.2) |
19 (42.2) |
122 (69.7) |
Chronic diseases |
|||
Yes |
33 (25.4) |
41 (60.3) |
74 (42.3) |
No |
97 (74.6) |
4 (8.9) |
101 (57.7) |
Asthma |
16 (12.3) |
5 (11.1) |
21 (12.0) |
Cardiovascular |
7 (5.4) |
19 (42.2) |
26 (14.8) |
COPD |
4 (3.1) |
17 (37.8) |
21 (12.0) |
Diabetes |
3 (2.3) |
8 (17.8) |
11 (2.3) |
Chronic kidney failure |
2 (1.5) |
4 (8.9) |
6 (3.4) |
Neurological/neuromuscular |
2 (1.5) |
5 (11.1) |
7 (4.0) |
Neoplasm |
1 (0.8) |
4 (8.9) |
5 (2.9) |
Other (malnutrición) |
1 (0.8) |
0 |
1 (0.6) |
Use of antiviral drugs |
|||
Yes |
1 (0.8) |
0 |
1 (0.6) |
No |
129 (99.2) |
45 (100) |
174 (99.4) |
Vaccination |
|||
Yes |
28 (21.5) |
1 (2.22) |
29 (16.6) |
No |
96 (73.9) |
38 (84.45) |
134 (76.6) |
NA |
6 (4.6) |
6 (13.33) |
12 (6.8) |
ICU admission |
|||
Yes |
7 (5.4) |
5 (11.1) |
12 (6.9) |
No |
123 (94.6) |
40 (88.9) |
163 (93.1) |
Mechanic ventilation |
|||
Yes |
5 (3.9) |
5 (11.1) |
10 (5.7) |
No |
125 (96.1) |
40 (88.9) |
165 (94.3) |
Death |
|||
Yes |
2 (1.5) |
3 (6.7) |
5 (2.9) |
No |
128 (98.5) |
42 (93.3) |
170 (97.1) |
Table 1: Demographic and clinical characteristics of the patients included in the study. FHINFP: Fundacion Hospital Infantil Napoleon Franco Pareja, HUC: Hospital Universitario del Caribe, ICU: Intensive Care Unit.
Additionally, severity was detected in 6.9% (n = 12) of the patients and they were admitted to Intensive Care Units (ICU), of which 5.7 (n = 10) received mechanical ventilation and 2.9% (n = 5) passed away).
Virological analysis and frequency of pathogensThe samples were processed to identify the influenza virus, other respiratory viruses (ORV) and 3 bacteria, the percentage of positivity was 59.4% (n = 104), RSV with 47.1% (n = 49), rhinovirus 33.6% (n = 35) and Influenza A with 15.4% (n = 16) were the most frequent pathogens (Figure 2). Some of these pathogens were found combined in coinfections; 4.8% (n = 5) of the patients had coinfection with three agents (RHV/VPIH/Bordetella Pertusi, RHV/VSR/AAV, RHV/VSR/Infl A, RHV/MPVH/Chlamydophila and RHV/AAV/Mycoplasma pneumoniae), and 13.5% (n = 14) coinfection with two agents (RHV/Chlamydophila, RHV/VPIH, VSR/CoVHKU1, RHV/VPIH, RHV/VSR, VSR/Infl A, RHV/VSR, VSR/CoVHKU1, RHV/RSV, Infl A/Chlamydophila, RSV/AAV, RHV/Chlamydophila, RHV/MPVH and VPIH/CoVHKU1, rhinovirus was identified as the first source of coinfection. Additionally, the frequencies of RSV and RHV were higher in FHINFP compared to HUC, p < 0.05.
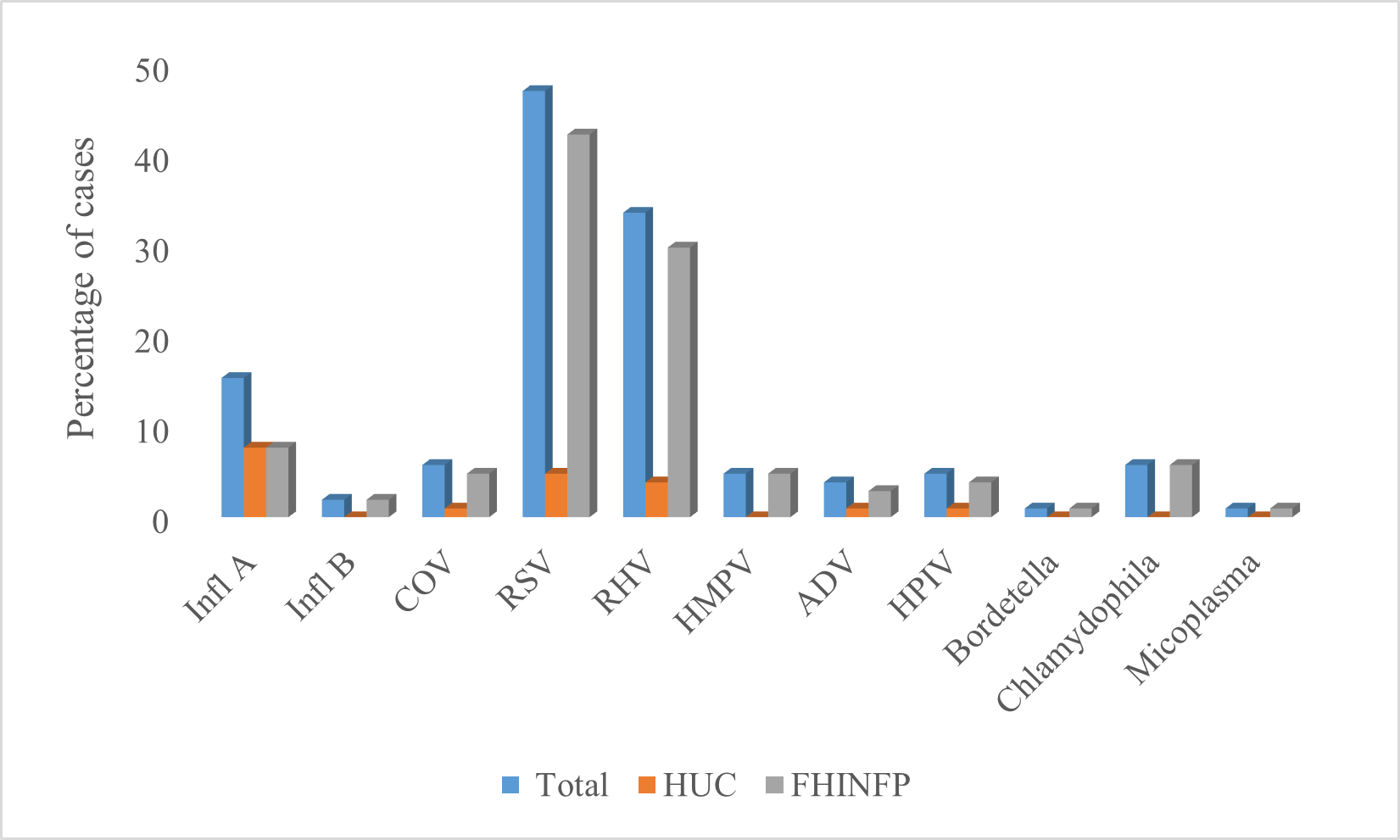
Figure 2: Frequency of pathogens; Influenza A: Infl A, Infl B: Influenza B, COV: Coronavirus, RSV: Respiratory Syncytial Virus, RHV: Rhinovirus, ADV: Adenovirus, HMPV: Human parainfluenza virus. (*) Statistically significant differences, p < 0.05.
As shown in table 2, 5.6% (n = 1) of influenza-positive patients were admitted to the ICU, received mechanical ventilation, and their outcome was fatal. Additionally, of those positive for other respiratory viruses, none were admitted to the ICU or died. The highest frequency of infection was given by other respiratory viruses and the presence of comorbidities was not significant (p = 0.952). In positive cases of influenza, antiviral therapy was not prescribed and vaccination was present in 16.7% (n = 3) of cases, fever was present in 61.1% (n = 11) and only the frequency of cases per hospital was statistically significant (p = 0.04). Influenza cases were distributed in age groups, while cases for other respiratory viruses were mainly concentrated in patients <2 years (p = 0.209).
| Characteristics | Positive n = 104(%) | Inf + n = 18(%) | ORV n = 86(%) | p |
|---|---|---|---|---|
Hospital |
||||
HUC |
19 (18.3) |
8 (44.4) |
11 (12.8) |
0.04 |
HINFP |
85 (81.7) |
10 (55.6) |
75 (87.2) |
|
Sex |
||||
M |
66 (63.5) |
13 (72.2) |
52 (60.5) |
0.308 |
F |
38 (36.5) |
5 (27.8) |
34 (39.5) |
|
Fever >38°C |
||||
Yes |
42 (40.4) |
11 (61.1) |
31 (36.1) |
0.066 |
No |
62 (59.6) |
7 (38.9) |
55 (63.9) |
|
Age group (years) |
||||
<2 |
64 (61.5) |
6 (33.3) |
58 (67.4) |
0.209 |
2-4 |
15 (14.4) |
2 (11.1) |
14 (16.3) |
|
5-17 |
5 (4.9) |
2 (11.1) |
2 (2.3) |
|
18-60 |
10 (9.6) |
3 (16.7) |
7 (8.2) |
|
>60 |
10 (9.6) |
5 (27.8) |
5 (5.8) |
|
Exposure to smoking |
||||
Yes |
8 (7.7) |
2 (11.1) |
6 (7.0) |
0.889 |
No |
85 (81.7) |
12 (66.7) |
73 (84.9) |
|
Ex-smoker |
11 (10.6) |
4 (22.2) |
7 (8.1) |
|
Chronic diseasess |
||||
Yes |
35 (33.6) |
11 (61.1) |
25 (29.1) |
0.952 |
No |
69 (66.4) |
7 (38.9) |
61 (70.9) |
|
Use of antiviral drugs |
||||
Yes |
1 (0.96) |
0 |
1 (1.2) |
NA |
No |
103 (99.04) |
18 (100) |
85 (98.8) |
|
Vaccination |
||||
Yes |
25 (24.0) |
3 (16.7) |
22 (25.6) |
0.215 |
No |
69 (66.4) |
13 (72.2) |
55 (63.9) |
|
NA |
10 (9.6) |
2 (11.1) |
9 (10.5) |
|
ICU Admission |
||||
Yes |
6 (5.78) |
1 (5.6) |
5 (5.8) |
0.803 |
No |
98 (94.2) |
17 (94.4) |
81 (94.2) |
|
Mechanic ventilation |
||||
Yes |
5 (4.8) |
1 (5.6) |
4 (4.7) |
0.803 |
No |
99 (95.2) |
17 (94.4) |
82 (95.3) |
|
Death |
||||
Yes |
1 (0.96) |
1 (5.6) |
0 |
NA |
No |
103 (99.04) |
17 (94.4) |
86 (100) |
|
Table 2: Clinical and epidemiological characteristics of patients with a positive diagnosis.
Infl +: Positive for influenza, ORV +: Positive for Other Respiratory Viruses, NA. Does not apply. ICU: Intensive Care Unit.
GIHSN was established globally with the goal of improving understanding of influenza infections and preventing morbidity and mortality in people at high risk for complications prematurely [13]. The information collected by this network in each country or region can contribute to the knowledge of the frequencies of infection by influenza and other respiratory viruses, the status of immunization by vaccination and implementation of efficient containment measures in endemic and high-risk areas such as countries low-income [3,13,15]. This is the first study to describe the frequency of influenza and other respiratory viruses in Cartagena-Colombia associated with SARI in adult and pediatric populations.
Patients of different ages who presented a comorbidity frequency of 42.3% in the general population (Table 1) and 34.6% in positive patients (Table 2) were included in the study, being asthma, cardiovascular disease, and obstructive pulmonary disease (COPD), diabetes, neurological/neuromuscular disease and chronic kidney failure are the most common comorbidities related to serious and fatal outcomes in pediatric and adult patients [3,16,17]. In the pediatric population, the association of influenza with these comorbidities causes recurrent wheezing [18], while in adults the infection can progress to pneumonia and severe acute respiratory distress (ARDS) [10,19].
In general, the frequency of positivity for influenza was 17.3%, (Figure 2), of these cases 88.9% were influenza A (72.2% AH3N2, 5.6% AHIN1 and 11.1% Influenza A without lineage) and 11.1% Influenza B. The association between influenza positivity (Table 2), comorbidities and mortality, it showed that 5.6% of deceased patients had cardiovascular disease and had an influenza B infection, the frequency of influenza mortality found in this study agrees with the data reported by the CDC [20]. However, our data and the information obtained on this association is limited by the low rate of positivity and mortality in the population studied regarding Influenza. RSV was the most frequent viral agent (Figure 2), an aspect similar to that previously reported in studies that included patients of all ages in Latin America, Europe, Asia, and Africa [4,15,16,21], although the real burden of RSV it could be higher, since the ILI and SARI inclusion criteria focus primarily on febrile symptoms, and many RSV patients often do not have fever [15]. RSV and rhinovirus can be considered the two infectious entities that cause SARI in Cartagena and Colombia [9], without ignoring the negative impact generated by the other viruses of less frequency [17,22].
Additionally, it was found that rhinovirus was the pathogen most prone to coexistence, because it was present in 72.2% of the co-infections and, curiously, bacterial co-infections occurred in the pediatric population <5 years, considering that children are more prone to mixed infections and according to our data mainly for Chlamydophyla, no evidence was found on more serious or lethal outcomes due to the coexistence of two or three infectious agents [23]. In addition, there was no difference in the clinical outcomes of patients who presented virus/virus or virus/bacteria co-infection compared to those who had a single viral infection (p > 0.05), so it could be assumed that there was no co-infection synergy between pathogens and that, on the contrary, there was competition for the cellular resource, decreasing the probability of a fatal outcome [24].
The immunity status against influenza by vaccination was relatively low in the study population (Table 1 and 2), acting as an important risk factor against influenza virus infection and serious outcomes of this pathology [23,24]. However, despite the low vaccination rate, the frequency of influenza and fatal outcomes in Cartagena was low. 76.6% of the patients reported not being vaccinated, indicating that the lack of access to the vaccine and the poor perception of its effectiveness and safety are some of the limitations for its application. Additionally, by relating the low academic training found in the adult population included in the study and the companions of the pediatric population, it could be stated that the poor perception of vaccination and the low frequency of vaccination would be associated with the poor educational level of the population [25,26].
Finally, the use of antivirals both in the general population and in patients positive for influenza and other respiratory viruses was significantly low (Table 1 and 2), this could be an indication of the low level of suspicion of viral infection in FHINFP hospitals and HUC, evidencing the overestimation of bacterial infections and misuse of antibiotics that has led to selective bacterial resistance [27]. Therefore, it is necessary to incorporate techniques such as multiple PCR (FilmArray), (RT-qPCR) and multi-pathogenic respiratory panels into clinical diagnostic tools, to obtain a timely and effective diagnosis in medical and emergency services, this could decrease the overuse of antibiotic therapy [27].
This study had some limitations; Among the most important, the small sample size stands out, there was a predominance of pediatric patients in the study population, a low number of patients in HUC and the burden of bacterial superinfection could not be assessed in positive cases for influenza, RSV and others virus. Despite all this, our results provide valuable information on the application of the active surveillance model against influenza and other respiratory viruses in Cartagena. Also, the frequency of each circulating virus and its relationship with serious outcomes were reported, the low use of antiviral therapy and the status of immunization against influenza by vaccination were also reported.
The authors thank the University of Cartagena and the Global Influenza Hospital Surveillance Network Foundation (GIHSN) for all the financial support granted during the course of the investigation.
The authors declare that they have no conflict of interest.
Citation: Doris Gómez-Camargo. “Global Influenza Hospital Influenza Surveillance Network (GIHSN), Results of Surveillance for Influenza and Other Respiratory Viruses in Cartagena-Colombia. Preliminary Data 2019-2020". Acta Scientific Microbiology 4.4 (2021): 39-45.
Copyright: © 2021 Doris Gómez-Camargo. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.