SD Khimich1*, FT Muravyev2, AP Prevar1, OO Olkhomiak1 and AV Funikov1
1National Pirogov Memorial Medical University, Vinnytsia, Ukraine
2Municipal Institution “O. F. Herbachevsky Regional Clinical Hospital”, Zhytomyr, Ukraine
*Corresponding Author: SD Khimich, National Pirogov Memorial Medical University, Vinnytsia, Ukraine.
Received: September 20, 2024; Published: October 11, 2024
Citation: D Khimich., et al. “Technical Difficulties in Laparoscopic Interventions for Complicated Forms of Gallstone Disease in Patients with Liver Cirrhosis". Acta Scientific Gastrointestinal Disorders 7.11 (2024):25-29
The paper focuses on surgical difficulties in laparoscopic interventions in patients with complicated forms of cholelithiasis and liver cirrhosis. Performing laparoscopic surgical interventions in patients with liver cirrhosis at a reduced level of carboxyperitoneum pressure (at a level of no more than 8-10 mm Hg), introducing the first trocar of paracentesis), insertion of the first trocar paraxiphoid to the right of the sword-shaped processby the Hasson method and additional 1-2 trocar multidirectional insertion, which is carried out through the tortuous channel of the trocar wound, by making 3-5 punctures and movements in the soft tissues at different angles, made it possible to significantly improve the technique of performing biliary surgery in patients with liver cirrhosis.
Keywords: Laparoscopic Interventions; Laparoscopic Cholecystectomy; Gallstone Disease; Cholecystitis; Liver Cirrhosis; Ascites; Intraoperative Period; Postoperative Period
In recent years, with the development of minimally invasive technologies, laparoscopic interventions have become the “gold standard” for the surgical treatment of gallstone disease, including complicated forms. Laparoscopic cholecystectomy (LCE) has become one of the most frequent operations and has become routine. However, concomitant liver cirrhosis significantly increases the risk of complications and forces doctors in many cases to refuse to perform LCE in favor of traditional cholecystectomy (CE).
At the same time, given the rapid development of minimally invasive technologies, modern surgery has managed to significantly reduce the percentage of postoperative complications, which cannot be said about the treatment of patients with concomitant liver cirrhosis (LC). The risk of postoperative complications and mortality in patients with liver cirrhosis is significantly higher and can reach 45% [1-7].
In addition, the relevance of geriatrics in emergency surgical practice is becoming more and more important every year and requires separate approaches to diagnosis, treatment tactics, management of the perioperative period and prevention of possible complications [8].
Thus, the treatment of complicated forms of cholelithiasis in the setting of liver cirrhosis (especially in geriatric patients) is not an easy task for the entire medical team.
The aim of our work was to identify the main difficulties in laparoscopic interventions during elective biliary surgery in patients with liver cirrhosis.
The work is based on the results of the analysis of clinical, laboratory and instrumental research methods, own observations and surgical treatment of complicated forms of cholelithiasis in patients with liver cirrhosis.
The prospective group (main group) included 41 patients who were treated with the updated algorithm for the treatment of this category of patients. The retrospective group (comparison group) included 38 patients whose treatment was analyzed according to the data from the inpatient medical records. The control group consisted of patients with cholelithiasis but without concomitant liver cirrhosis (113 patients), whose treatment was analyzed to determine the risk predictors of treatment of patients with concomitant liver cirrhosis. There was no statistically significant difference in the age of patients in the study groups. In the main group, the age of patients was 62.8 ± 3.9 years, and in the comparison group - 56.0 ± 3.5 years at p>0.05. In patients of the main and control groups, the etiologic factors of liver cirrhosis were determined. Thus, in both groups of the study, the main cause of cirrhosis was infection with viral hepatitis, which amounted to 69 cases, alcoholic cirrhosis was diagnosed in 9 patients, cardiogenic cirrhosis in 1. Patients of both groups (main and comparison groups) at the time of hospitalization underwent cirrhosis staging according to the Child-Turcotte-Rugg system. In patients of the main group, the number of points according to the MELD (Model of Endstage Liver Disease) system was additionally determined. All patients after the verified main diagnosis according to the improved and standard algorithm were prescribed etiologic and pathogenetic conservative therapy, which was aimed at detoxification, antibacterial action, assessment and implementation of thromboprophylaxis and nutritional support. In the comparison group, all patients received standard regimens for the prevention of purulent-septic complications and thromboprophylaxis. Patients in the main group were divided into two cohorts according to the risk of hemorrhagic and embolic complications, based on the prevalence of bleeding and embolization risks, with the aim of prescribing a standard thromboprophylaxis regimen and early postoperative prophylaxis. During antibiotic therapy in patients of the main group, antimicrobial prescriptions were based on the principles of early de-escalation therapy and, if possible, limiting the duration of antimicrobial therapy to reduce the risk of hepatitis in patients with compromised liver function.
In the treatment of complicated forms of cholelithiasis in the setting of liver cirrhosis, a clear choice of treatment methodology (laparotomy or minimally invasive laparoscopic approach) is quite important.
Performing laparoscopic interventions in the presence of ascites is accompanied by an increased risk of purulent-septic complications from postoperative wounds due to leakage of ascitic fluid, so in the treatment of patients in the main group, the method of introducing trocars in patients with concomitant liver cirrhosis and ascites according to our method was used.
According to the analysis of the effectiveness of the introduced diagnostic and therapeutic algorithm and a comprehensive assessment of the treatment results of both groups of patients, the following data were obtained. In the main group of patients, the average length of stay in the clinic was 7.37 ± 0.46 days, in the control group - 8.42 ± 0.55 days (p > 0.05). The difference in the duration of the operation and the volume of intraoperative blood loss by group was statistically significant. Thus, in the main group, the average duration of the operation was within 96.2 ± 10.1 minutes, and the volume of blood loss was 101.1 ± 12.5 ml, while in patients of the control group the indicators were 115.5 ± 9.7 minutes and 155.2 ± 20.5 ml (p ≤ 0.05). The main indicators of the effectiveness of the used diagnostic and therapeutic program were the assessment of perioperative complications and postoperative mortality. Thus, in the main study group, postoperative complications occurred in 6 cases (14.6%), while in the comparison group, complications were detected in 13 (34.2%), which was statistically confirmed at p ≤ 0.05 (χ2 = 4.14). According to the Clavien-Dindo classification, complications of class III B (2 cases in the main group; 3 cases in the comparison group), IV A (5 cases in the comparison group) and V classes prevailed in the control group. In 3 patients of the comparison group, death occurred, which was reflected in the Clavien-Dindo classification as class V. At the same time, in the main group of patients, 1 patient died.
Until recently, LCE in patients with liver cirrhosis was considered quite controversial and very risky. Performing operations in cirrhotic liver with portal hypertension requires high qualification of surgeons, availability of material and technical support of the hospital to correct the negative manifestations of cirrhosis.
The main factors for performing effective laparoscopic interventions for concomitant LC are:
All doctors involved in the treatment process of this category of patients face many factors that need to be addressed, both during surgery and in the postoperative period. The main factors that determine the complexity of laparoscopic interventions for liver cirrhosis are as follows:
LCE in complicated forms of biliary diseases is one of the most commonly performed biliary operations. Standard indicators of carboxyperitoneum pressure in the abdominal cavity during these operations are 12-14 mm Hg. Despite the proven safety of this pressure level, patients with cirrhosis are more prone to a decrease in organ blood supply at such insufflation rates, which leads to worsening of compromised liver function in cirrhosis, and deterioration of cardiac contractility.
We analyzed the effect of carboxyperitoneum on cardiac contractile function and identified a group of patients intolerant to standard CO2 insufflation. Pulse rate and systolic blood pressure were monitored after 1, 5 and 15 minutes. The comparative assessment is shown in the graphs (Figure 1 and 2). At the beginning of surgery, patients in the main and control groups had similar heart rate and systolic blood pressure (SBP) values. Thus, SBP values were 121.4 ± 10.2 and 121.8 ± 9.1 mm Hg, respectively. At 1 minute of insufflation, an increase in blood pressure was observed in patients of both groups.
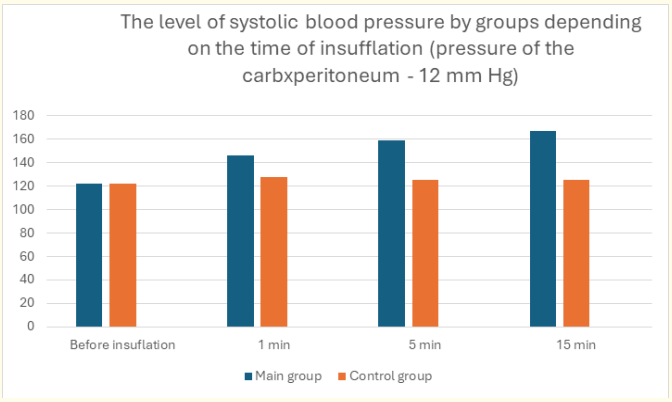
Figure 1: Level of systolic blood pressure by groups depending on the time of insufflation (carboxiperitoneum pressure - 12 mm Hg).

Figure 2: Pulse rate by groups depending on the time of insufflation.
Subsequently, patients in the control group showed stabilization of SBP and pulse, and in the main group, patients showed a steady increase in both SBP to 167.4 ± 12.8 mm Hg (compared with the main group - 125.3 ± 11.9 mm Hg) and pulse rate: the main group - to 129 ± 14.2, the control group - to 92 ± 15.6.
As can be seen from the above results, in patients with LC, the increase in systolic blood pressure after 15 minutes increased by 27.0% of the initial level, and the pulse rate by 32.5%. At the same time, in patients of the control group - only by 8.7%. Given this rather significant increase in SBP, we decided that laparoscopic surgical interventions should be performed at a reduced level of carboxyperitoneum pressure (at a level of no more than 8-10 mm Hg). Intraoperative monitoring of the patient's blood acid-base status should be performed if the operation lasts more than 1.5 hours. In all cases, the decision to perform laparoscopic interventions is made by a commission with the participation of a surgeon and an anesthesiologist. At the beginning of the operation, the patient's tolerance to carboxyperitoneum pressure is tested. In the absence of a pronounced negative reaction, the operation is performed laparoscopically. Laparoscopic surgical interventions at reduced pressure in the abdominal cavity were performed both in the main (n = 29) and in the control group (n = 19). The main indications for surgery at a carboxyperitoneum pressure in the abdominal cavity of 8-10 mm Hg in the control group were severe cardiac pathology (combined aortic disease, mitral valve disease, chronic coronary heart disease with heart failure and reduced left ventricular ejection fraction).
Portal hypertension is a manifestation of liver cirrhosis. The formation of venous collaterals is due to both increased angiogenesis and parenchymal blockage of venous flow in the portal vein basin. With the progression of LC, especially in the decompensated stage, venous collaterals of the “jellyfish head” type are formed on the abdominal wall. Damage to these collaterals leads to massive bleeding, and stopping the bleeding is achieved in a very difficult way. In our study, dilated veins of the anterior abdominal wall were observed in 14 patients (34.14%) of the main group. According to the stage of cirrhosis, they corresponded to stages B (n = 12) and C (n = 2) according to Child Pugh. In order to avoid damage to the abdominal wall veins and in all patients with dilated abdominal wall veins, the first trocar was inserted paraxiphoidally to the right of the sword-shaped process.
Another important aspect of performing laparoscopic interventions in patients with LC is the traction of the gallbladder in the cranial direction. Traction of the gallbladder by the bottom allows you to carefully identify the elements of the Calot's triangle and reduce the risk of damage outside the hepatic bile ducts. However, due to cirrhotic changes, the mobility of the liver is significantly limited. To minimize the inconvenience of reduced liver mobility, we proposed the LCE technique using 5 trocars. In addition to the generally accepted positions of 3-4 trocars, we additionally introduced a 5 mm trocar in the left hypochondrium along the anterior clavicular line to introduce a hepatic retractor of the “gold finger” type. After the trocar was inserted, the liver's circular ligament was transected to promote greater liver mobility. The scheme of trocars is shown in Figure 3.
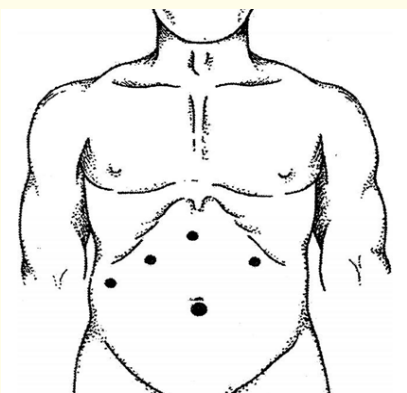
Figure 3: Scheme of trocar placement.
The improved LCE technique with the use of 5 trocars was used in 17 patients of the main group with acute calculous cholecystitis and in 5 patients of the control group. The introduction of an additional trocars in patients without concomitant liver cirrhosis was due to severe obesity and hepatomegaly.
One of the peculiarities of performing surgeries for complicated forms of GI disease in the setting of liver cirrhosis is the operation in the setting of ascites. In the postoperative period, many patients experience ascitic fluid leakage from trocar wounds due to the lack of trocar wound sealing during laparoscopic interventions in the setting of ascites. In order to prevent such complications and improve the healing of postoperative wounds, we have developed and implemented a method of multidirectional insertion of trocars into the abdominal cavity. With this technique, the trocar is inserted through the twisted channel of the trocar wound, by making 3-5 punctures and movements in the soft tissues at different angles. This leads to the fact that after removal of the trocar, the soft tissues take their position, overlap one layer with another and the wound closes, which subsequently prevents the leakage of ascitic fluid.
Thus, laparoscopic surgical interventions in patients with LC at a reduced level of carboxyperitoneum pressure (at a level of no more than 8-10 mm Hg), insertion of the first trocar paraxiphoid to the right of the sword-shaped process by the Hasson method and additional 1-2 trocar of multidirectional insertion, which is carried out through the tortuous channel of the trocar wound, by making 3-5 punctures and sinuous movements in the soft tissues at different angles, made it possible to significantly improve the technique of performing biliary surgery in patients with cirrhosis.
Copyright: © 2024 SD Khimich.,et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.