Anubha Bajaj*
Department of Histopathology, Panjab University/A.B. Diagnostics, India
*Corresponding Author: Anubha Bajaj, Department of Histopathology, Panjab University/A.B. Diagnostics, India.
Received: September 04, 2024; Published: October 01, 2024
Citation: Anubha Bajaj. “Spoked and Cicatrix- Radial Scar/Complex Sclerosing Lesion- Breast". Acta Scientific Gastrointestinal Disorders 7.11 (2024):01-03.
Radial scar or complex sclerosing lesion delineates a benign breast lesion articulating stellate configuration. Tumefaction is constituted of a centric core of fibro-elastotic stroma intermingled with radiating ducts and lobules along with proportionately variable proliferative and cystic glandular alterations. Upon clinical examination, gross evaluation, histological assessment and mammography, neoplasm may recapitulate invasive carcinoma breast. Focal cellular and nuclear atypia or malignant metamorphosis may arise within or in concurrence with radial scar/complex sclerosing lesion.
Additionally designated as radial sclerosing lesion or complex sclerosing lesion, tumour magnitude commonly exceeds > 1.0 centimetre [1,2]. Inadvertently arising within the breast parenchyma, lesion emerges between 40 years to 70 years. Tumefaction demonstrates an equivocal frequency of detection between surgical samples obtained for assessing benign and malignant breast lesions. Frequently, neoplasm appears as a multi-centric, bilateral lesion [1,2]. Of obscure aetiology and pathogenesis, neoplasm is posited to arise due to stromal-epithelial interaction and demonstrates enhanced expression of diverse factors implicated in configuration of vascularized stroma. However, terminology of scar emerges as a misrepresentation as contributory factors as preceding trauma or surgical intervention are absent [2,3].
The lesion expounds focal augmentation of quantifiable vascular articulations. Besides, expression of mRNA for collagen type I, total fibronectin, ED-A+ fibronectin, thrombospondin 1, vascular endothelial growth factor or vascular permeability factor (VEGF/VPF) and angiogenic receptor(KDR) is observed. Allelic imbalance within chromosome 16q and 8p may ensue. Frequently, tumour zones appear clone specific [2,3]. Clinically, majority of lesions are discerned incidentally upon microscopic assessment of breast tissue excised for various anomalies or target lesions. Nevertheless, few palpable lesions may be encountered [2,3]. Fine needle aspiration cytology (FNA) appears unreliable for discerning radial scar or complex sclerosing lesions. Generally, foci of usual ductal hyperplasia and apocrine metaplasia may appear commingled with spindle shaped stromal cells [3,4].
Grossly, tumefaction configures a stellate, rubbery to firm mass with a retracted centric zone. Neoplasm may simulate invasive ductal carcinoma. Commonly, radial scar is ≤ 1 centimetre diameter whereas complex sclerosing lesions appear to exceed > 1 centimetre magnitude. Cut surface appears firm, irregular and depicts yellow streaks or flecks on account of intermingled elastotic stroma [3,4]. Upon low power microscopy, lesion expounds a stellate configuration. Centric sclerotic zone delineates focal fibrosis and elastosis with commingled ducts and lobules demonstrating peripheral radiation between fascicles of sclerotic tissue. Frequently, centric core is impregnated with entrapped, miniature, obliterated ductules, a feature which appears reminiscent of invasive carcinoma. The centric tissue nidus may enunciate foci of squamous metaplasia. Lesion exemplifies quantifiably variable foci of usual ductal hyperplasia or florid ductal hyperplasia admixed with focal necrosis, sclerosing adenosis, apocrine metaplasia or an amalgamation of cysts [3,4]. Circumscribing stroma encountered within preliminary lesions is significantly cellular. Ancient lesions appear collagen-rich or sclerotic and are pervaded with abundant elastin fibres. Up to 51% lesions are concordant with foci of atypical hyperplasia as atypical ductal hyperplasia (ADH) or atypical lobular hyperplasia (ALH). Foci of in situ or invasive carcinoma may be confined to or appear in concurrence with radial scar or complex sclerosing lesions [3,4]. Characteristically, enlarged complex sclerosing lesions > 1 centimetre magnitude delineate features of radial scar intermingled with zones of sclerosis, entrapped and distorted glandular articulations and fibrocystic change. Notwithstanding, lesion may lack the well defined configuration of radial scar [3,4].
![Figure 1: Radial scar demonstrating glandular structures layered with ductal epithelial cells intermingled with foci of usual ductal hyperplasia and surrounding collagen-rich, sclerotic stroma [7].](https://actascientific.com/ASGIS/images/ASGIS-07-0673_figure1.png)
Figure 1: Radial scar demonstrating glandular structures layered with ductal epithelial cells intermingled with foci of usual ductal hyperplasia and surrounding collagen-rich, sclerotic stroma [7].
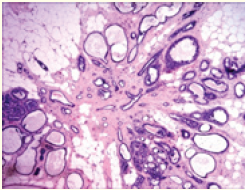
Figure 2: Level of co-localization between mRFP-Vps1 domains and Gga1-GFP. (A) mRFP- Vps1 full length and its truncated species in the first row. Gga1-GFP in the same strain in the second row. The third row shows the merged image. (B) Percentage of cells showing colocalization of mRFP-Vps1 domain to Gga1-GFP carrying endocytic dots.
![FTable a: Benign breast lesions concomitant with radial scar [1].](https://actascientific.com/ASGIS/images/ASGIS-07-0673_table1.png)
Table a: Benign breast lesions concomitant with radial scar [1].
Radial scar or complex sclerosing lesion appears immune reactive to myoepithelial markers as p63, calponin, CD10, smooth muscle myosin heavy chain, smooth muscle actin, CK5 or various elastic stains. Centric tumour zone displays attenuated or absent myoepithelium. Nevertheless, immune reactivity to smooth muscle myosin heavy chain, calponin and p63 may be encountered [4,5]. Radial scar or complex sclerosing lesion requires segregation from neoplasms as invasive ductal carcinoma, tubular carcinoma or low grade adeno-squamous carcinoma [4,5]. Neoplasm may be appropriately ascertained with diverse imaging techniques as ultrasonography, mammography or magnetic resonance imaging (MRI). Generally, invasive diagnostic procedures as core needle biopsy or fine needle aspiration cytology are not recommended. Upon mammographic imaging, tumefaction may depict architectural distortion or appear spiculated or may simulate invasive carcinoma. Lesion appears uniformly dense. Centric zone may be radiolucent. Focal micro-calcification may be exceptionally observed. Characteristically, features such as cutaneous thickening or cutaneous retraction appear absent. Radial scar is devoid of a distinct scirrhous reaction [4,5]. Ultrasonography expounds an irregular, poorly defined, hypoechoic tumefaction with inadequately defined perimeter and posterior acoustic shadowing. Generally, lesion appears spherical, elliptical or lobulated. Variable intrinsic echoes may be exemplified. Few radial scars may delineate retro-acoustic attenuation [4,5]. Magnetic resonance imaging (MRI) displays variable characteristics of image enhancement. Benign lesions delineate absence of image enhancement wherein image enhancement is indicative of concordant emergence of malignant neoplasms. Additionally, the lesion exhibits spiculation and architectural distortion [4,5]. Therapeutic strategies may vary from simple observation in association with meticulous clinical and radiological monitoring or surgical extermination of the lesion may be employed. As the treatment strategies vary from surgical eradication to simple clinical observation, proportionate discernment of malignant neoplasms upon surgical excision appears variable. Radial scar devoid of cellular and nuclear atypia demonstrates mean malignant metamorphosis in ~7% lesions although up to 16% neoplasms may be affected [4,5]. Minimal proportionate occurrence of malignant metamorphosis may not warrant surgical extermination, especially in lesions where concurrence of clinical monitoring with radiographic and histopathological assessment is adopted. Few incidentally discovered, miniature radial scars < 5 millimetre magnitude may depict malignant transformation within surgical excision specimens. Lesions delineating cellular atypia may be appropriately managed with strategies applicable for concurrent high risk lesion [4,5]. Vacuum assisted large core biopsy may be optimally adopted for excluding malignant transformation within a lesion wherein surgical eradication of the lesion may be circumvented. Factors contributing to emergence of invasive carcinoma breast appear debatable. Radial scar is non concurrent with enhanced possible occurrence of invasive carcinoma breast. Enlarged, multiple radial scars appear concordant with augmented possible occurrence of carcinoma breast by twofold [4,5]. Exceptionally, tumefaction may concur with adenosquamous carcinoma. Radial scar and complex sclerosing lesion exceeding > 10 millimetre diameter or lesions < 10 millimetre magnitude appear non concurrent with prognostic outcomes [4,5].
Copyright: © 2024 Anubha Bajaj. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.