Jose Orlando M Nicolas*, Ira Inductivo-Yu and Mara Teresa Panlilio
Department of Internal Medicine, National Kidney and Transplant Institute, Philippines
*Corresponding Author: Jose Orlando M Nicolas, Department of Internal Medicine, National Kidney and Transplant Institute, Philippines.
Received: August 02, 2024; Published: August 20, 2024
Citation: Jose Orlando M Nicolas., et al. “Experience on use of Double Plasma Molecular Adsorption System (DPMAS) Among Patients with Liver Failure and Decompensated Liver Cirrhosis in Tertiary Specialty Centers-A Case Series". Acta Scientific Gastrointestinal Disorders 7.9 (2024):29-34.
Background: Liver transplantation is the definitive management for acute liver (ALF) and end-stage liver disease (ESLD). This is limited by accessibility, lack of potential donors, and cost. At present, as a bridge for liver transplantation, artificial liver support systems (ALSS) such as double plasma molecular adsorption system (DPMAS) are being utilized.
Methods: This case series aimed to review outcomes of patients who had DPMAS from 2018 to 2023. This case series included patients from a tertiary referral center in the Philippines. Records of patients who had acute-on-chronic liver failure, acute liver failure, and severe decompensated liver cirrhosis who underwent DPMAS were reviewed.
Results: Ten patients were included in this series. Seven out of the ten patients were discharged, while three died from multi-organ failure. Prothrombin time and INR improved post-DPMAS. Consequently, prognostic scores, specifically MELD-Na, was lower. Likewise, Hepatic Encephalopathy (HE) grade and degree of ascites improved. Two of these patients underwent successful liver transplantation eventually.
Conclusion: Patients who underwent DPMAS in this series showed improvement in clinical as well as laboratory parameters measuring hepatic synthetic function, and MELD-Na. DPMAS is a promising modality for bridging ALF and end-stage liver disease to transplantation, and in some cases, recovery to compensated state.
Keywords: DPMAS; ACLF; Liver Failure; Prognosis; Liver Transplantation
Acute-on-chronic liver failure (ACLF) is a syndrome characterized by decompensation in patients with chronic liver disease, generally secondary to one or more extra-hepatic organ failures. Acute decompensation (AD), on the other hand, is used for one or more consequences of liver disease including hepatorenal syndrome, variceal bleeding, and Spontaneous Bacterial Peritonitis (SBP) in a short time [1]. The latter being the most common reason for hospital admission in patients with liver cirrhosis. In addition, acute liver failure (ALF) refers to the development of severe acute liver injury with impaired synthetic function (INR of ≥1.5) and altered mental status in a patient without cirrhosis or preexisting liver disease. The previously stated complications are associated with high mortality rate prompting urgent management.
Risk stratification systems among this subset of patients are needed to determine who likely will benefit more with treatment. One of the scoring systems used among this subset of patients is the Chronic Liver Failure Consortium ACLF (CLIF ACLF). It incorporates the number of failing organ systems, age and white cell count to calculate an ACLF score and a predicted mortality rate. The score is dynamic throughout a patient’s course [2].
Liver transplantation is the definitive management for liver failure, which is limited by accessibility, lack of potential donors, and cost [3]. At present, as a bridge for liver transplantation, artificial liver support systems (ALSS) such as double plasma molecular adsorption system (DPMAS) are being utilized [4].
ALSS serve as bridge to liver transplantation (i.e., while waiting for a suitable organ or to liver recovery) [5]. It removes bilirubin, cytokines, endotoxins, aromatic amino acids, and ammonia in the blood. Based on available literature, total plasma exchange (TPE) shows benefit over standard medical treatment in bridging patients to transplantation. It improves coagulopathy, bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), And ammonia [6]. Compared to plasma exchange, ALSS such as DPMAS has common advantages and lower adverse events including urticaria, infection, and shock while significantly improving patients’ prognosis [7].
DPMAS is one of the newer ALSS technologies. It uses HA330-Ⅱ Disposable Hemoperfusion Cartridge to adsorb primarily ammonia as well as medium and large molecular toxins, combined with BS330 Disposable Plasma Bilirubin Adsorption Column to adsorb bilirubin and bile acids. It has a good adsorption effect on bilirubin and inflammatory mediators without consuming plasma [6].
According to the study by Wu., et al. in 2021, DPMAS significantly improved transplant-free survival in patients with liver cirrhosis. After DPMAS treatment, significant decline was noticed in the total bilirubin, direct bilirubin, total bile acid, and cholesterol of patients [8]. Another study by Wan., et al. in 2017 concluded that DPMAS improves 12-week survival in patients with acute on chronic liver failure [6,9,10].
This case series presents the outcome of patients who underwent DPMAS in our institution.
This case series included patients admitted at Philippine Heart Center and at National Kidney and Transplant Institute, tertiary care institutions in the Philippines. The records of patients who had acute-on-chronic liver failure, acute liver failure, and decompensated liver cirrhosis who received DPMAS were reviewed. Patients included were adults aged 19 years old and above, patients who had acute-on-chronic liver failure, acute liver failure, and decompensated liver cirrhosis who underwent DPMAS therapy.
All patients received standard medical therapy (SMT) including bed rest, adequate nutritional support, enteral with or without parenteral nutrition, antiviral treatment for those with chronic hepatitis B, sodium restriction, diuresis, paracentesis for those with volume overload/ascites. Culture-guided antibiotics given. Hepatic encephalopathy regimen also started. Inotropic support also was given to patients during hypotensive periods.
All patients received DPMAS (HA 330II and BS 330 cartridge utilization), except for one patient who only had BS 330 cartridge hemoperfusion. All had triple intrajugular catheter as access, except for those who had femoral catheter. Continuous renal replacement therapy (CRRT) machine was used to facilitate DPMAS. A hospital nephrologist was called to comanage all patients during DPMAS sessions. Patients were hooked to the CRRT machine between 2-3 hours per session, and each session was spaced 2-4 days apart depending on the condition of the patient. Heparin was used for anticoagulation. Blood transfusion given for those with platelet < 75,000 (or if with active bleeding), PT INR > 1.5 (or if with active bleeding, or significant bruising) as stated in the institution’s released manual on blood purification through sorbent technology [12,13].
Descriptive statistics were used to summarize the data obtained. The research study adhered to the ethical considerations and principles defined by relevant guidelines. Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
J.C. is a diagnosed case of alcohol-related liver cirrhosis admitted for fever and managed as complicated urinary tract infection. During the hospital course, he had a MELD-Na score of 25, with HRS thus diagnosis of ACLF was made. In addition to standard medical management, he underwent DPMAS for three sessions. The degree of hepatic encephalopathy and ascites decreased after medical management with DPMAS sessions. Patient was discharged improved.
A.C. is a diagnosed patient with chronic hepatitis B for 20 years presenting with jaundice and decrease in sensorium. He had baseline MELD-Na score of 34, and initial CLIF score of 66. Upon admission, he was managed for ACLF with spontaneous bacterial peritonitis. DPMAS was done for 1 session. Patient was enlisted for liver transplantation, but due to lack of suitable deceased donor, he succumbed to multiorgan failure.
M.L. was diagnosed with Breast Invasive Ductal Carcinoma Stage IV with metastasis to the liver. Patient was ongoing palliative chemotherapy with paclitaxel and capecitabine when she presented with elevated bilirubin, ALT, PTT, creatinine, and increase in ascites. Chemotherapy was put on hold; diuresis and antibiotics were initiated. Despite these, she had deterioration of laboratory parameters. She then underwent 2 sessions of DPMAS. Post-DPMAS laboratory results showed improvement of the following liver function tests. She was discharged improved prior to resumption of palliative chemotherapy.
I.L. is a diagnosed patient with liver cirrhosis from MASLD advised for admission for bleeding esophageal varices. Baseline prognostic scores: Child Pugh Score C (14), MELD-Na 34 and CLIF 54. He was managed with upper endoscopy with rubber band ligation and initiation of vasoconstrictors. DPMAS was done while patient was being optimized for liver transplant. Comparative laboratory results before and after DPMAS showed: ALT of 44 U/L from 76 U/L, 13.2mg/dL from 6.6mg/dL, INR of 2.01 from 1.8 and ammonia of 87 umol/L from 116 umol/L. Despite an interval increase in bilirubin and INR attributed to sepsis, there was resolution of patient’s ascites and hepatic encephalopathy. MELD-Na and CLIF scores improved after DPMAS to 29 and 40, respectively. The patient underwent successful liver transplantation after a match with a suitable deceased donor.
The patient was admitted for ACLF from Chronic hepatitis B-related cirrhosis with complicated UTI as precipitating factor. He had the following prognostic scores on admission: MELD-Na 33, Child Pugh C (14) and CLIF 55. Baseline labs showed ALT of 90 U/L, total bilirubin of 21.9 mg/dL, INR of 3.09, and ammonia of 82 umol/L. He underwent DPMAS for 2 sessions while awaiting resolution of CUTI prior to liver transplant. There was interval decrease of ALT, bilirubin, and INR to 34 U/L, 14.97 mg/dL and 2.96, respectively. MELD-Na and CLIF scores improved to 31 and 47, respectively. He eventually underwent deceased donor liver transplantation and was discharged improved.
The patient was admitted for acute respiratory failure secondary to septic shock and acute kidney injury from Weils Syndrome. He was admitted to ICU and was put on extracorporeal membrane oxygenator (ECMO) support for acute respiratory distress syndrome (ARDS) from pulmonary hemorrhage. In addition to hemoperfusion to address cytokine storm, the managing team consensus was to initiate DPMAS therapy to prevent bile nephropathy from marked hyperbilirubinemia as an additional factor for kidney injury. After therapy, total bilirubin decreased to 24.6 mg/dL. However, patient had progressive multiple organ failure and expired.
Three patients had acute liver failure from ischemic hepatitis secondary to cardiogenic shock. Patient 9 had fatty liver at baseline. Upon assessment of liver failure, patients 7, 8, and 9 had baseline ALT levels as follow: 1727 U/L, 205 U/L, and 101 U/L, respectively. They all had grade 3 hepatic encephalopathy and hyperbilirubinemia which ranged from 2.05 mg/dL to 8.3 mg/dL. The level of serum ammonia before and after DPMAS showed consistent interval decrease. Patient 7 underwent 3 DPMAS sessions, while patients 8 and 9 underwent 2 sessions. Post-DPMAS, there was resolution of hepatic encephalopathy with improvement of MELD Na, Child-Pugh and CLIF scores. They were all discharged improved.
M.G. was diagnosed with liver cirrhosis from chronic hepatitis B. She had the following prognostic scores on admission: MELD-Na 27, Child Pugh C (10) and CLIF 59. Baseline labs showed ALT of 218 U/L, total bilirubin of 24.3 mg/dL, INR of 1.4, and ammonia of 384 umol/L. She had spontaneous bacterial peritonitis as her decompensating event. She underwent 2 DPMAS sessions. There was interval decrease in laboratory results after DPMAS (ALT of 100 U/L, total bilirubin of 15 mg/dL, and ammonia of 180 umol/L. INR was maintained at 1.4. However, patient had progressive septic shock and expired.
The patient characteristics and outcomes are shown in table 1. Ten patients were included in this study. The patients’ age ranged from 27 to 77 years old. Seven patients were male. Three patients presented with ACLF, while two other patients had an acute decompensating event of liver cirrhosis. In addition, one patient had acute liver failure related to metastatic breast cancer. Another patient had bile nephropathy complicating severe leptospirosis. Three patients had acute liver failure from ischemic hepatitis secondary to cardiogenic shock. The average DPMAS sessions from presentation to outcome are 1.8 sessions.
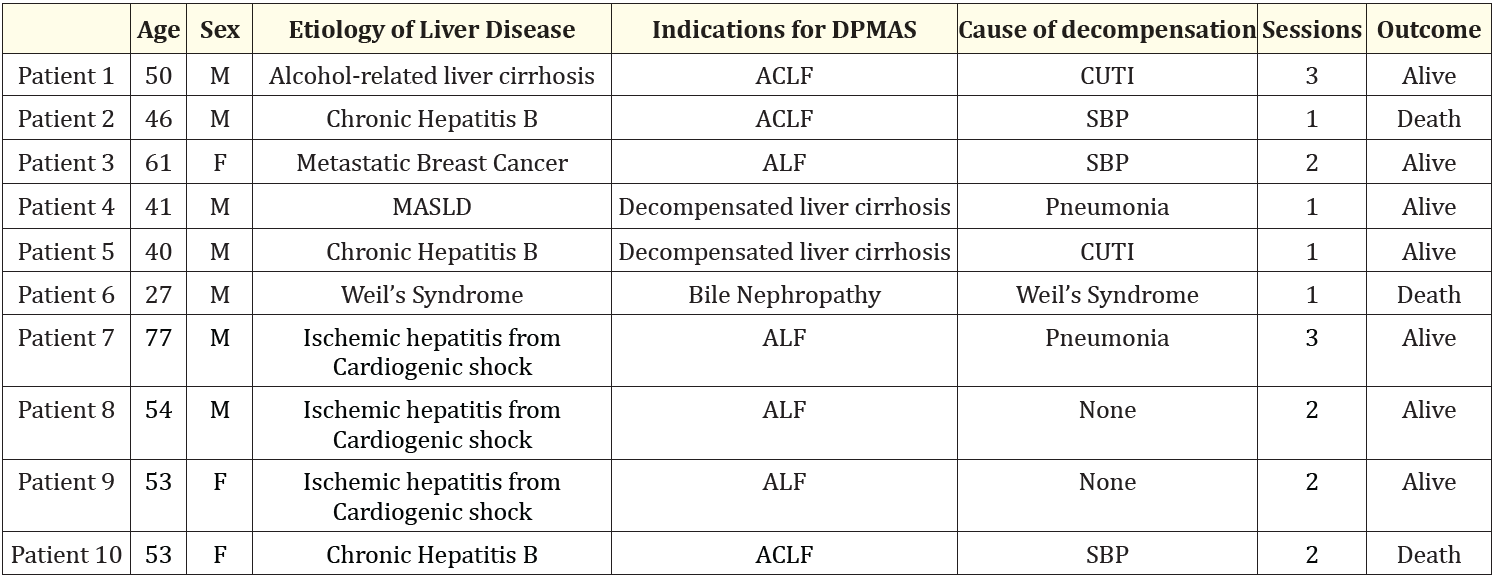
Table 1: Patient characteristics and outcomes. Abbreviations: MAFLD: Metabolic Associated Fatty Liver Disease; ALF: Acute Liver Failure; ACLF: acute on Chronic Liver Failure; CUTI: Complicated UTI; SBP: Spontaneous Bacterial Peritonitis
Eight patients had concurrent sepsis from varying causes which included complicated urinary tract infection, spontaneous bacterial peritonitis, severe leptospirosis, and hospital-acquired pneumonia. Among these patients, 5 were discharged improved. Two were bridged to liver transplant, while the remaining three patients died from multiorgan failure.
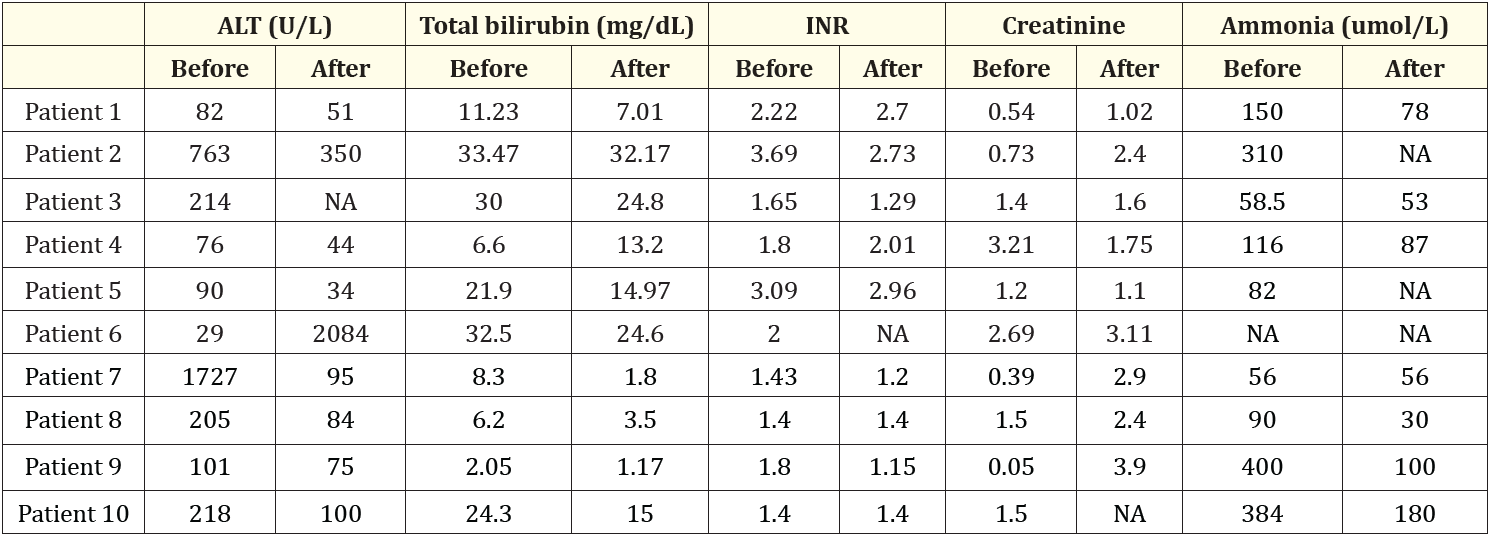
Table 2: The laboratory results between the peak values before DPMAS and after DPMAS. Abbreviations: ALT: Alanine Aminotransferase; INR: International Normalized Ratio; NA: Not Available
The laboratory results related to liver dysfunction including ALT, total bilirubin, INR, creatinine and ammonia before and after DPMAS treatment are summarized in table 2. The MELD-Na, CLIF ACLF, and CTP scores before and after DPMAS are noted in table 3.
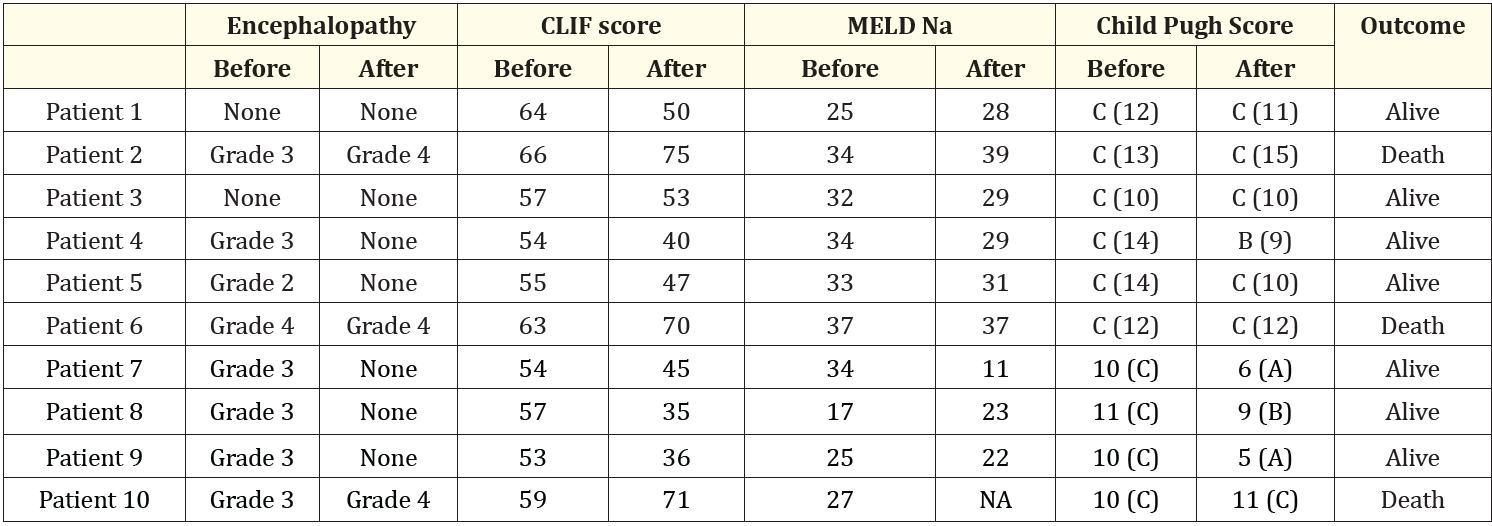
Table 3: Prognostic scores before DPMAS and after DPMAS. Abbreviation: NA: Not Available
In this case series, it is evident that the liver clearance function of patients with ACLF, ALF and severe decompensated liver cirrhosis was significantly reduced. The accumulation of endogenous toxic substances, such as various water-soluble toxin, protein-bound toxins, and metabolites, seriously affect hepatocyte regeneration and hence the recovery of liver function in these patients [14].
At present, liver transplantation is the definitive management for liver failure. However, this is limited by expertise, accessibility, lack of potential donors and cost [2]. As a bridge to liver transplantation, the artificial liver support systems (ALSS) are used [3]. ALSS removes excess bilirubin and bile acids, ammonia and other inflammatory mediators, and promotes recovery. An example of this ALSS is DPMAS.
In this case series, a third of the patients died due to sepsis and multi-organ failure. These patients had higher admitting MELD scores and worse HE grades. This contrasts with the 7 other patients with better baseline liver function. This is consistent with results from previous studies which showed that ALT, INR, bilirubin, and HE are predictors of mortality in patients with ACLF and ALF. Two patients (patients 4 and 5) were successfully bridged to liver transplantation with DPMAS. A study by Ling., et al. showed that DPMAS improved short-term survival in patients with ACLF through improved hepatic synthetic test results [11].
Likewise in a CART analysis done, patients were deemed to have high 28-day mortality risk if there is HE or if PT > 28 seconds even after ALSS therapy.16 The 3 mortalities in our case series had progression of their encephalopathy despite the DPMAS received.
Preliminary data from literature show promising use of DPMAS in ACLF and ALF. A meta-analysis by Zhang., et al. explored the efficacy and safety of DPMAS-based artificial liver support in the management of ACLF [5]. Similar to our series, the total bilirubin significantly decreased with DPMAS which was related to the specific BS330 bilirubin adsorption column in DPMAS [11]. Our observations also indicated that in addition to total bilirubin, there was also a decrease in ALT, serum ammonia, and creatinine with DPMAS. The proposed mechanism is clearance of inflammatory factors with this modality.
On the other hand, the improvement of INR were not observed consistently in this series. This is explained by the contact between the blood of patients and the adsorption column which damages the coagulation factors [6].
As expected, improvement of bilirubin levels and creatinine consequently improved the MELD-Na and CTP scores of the patients in this series. Improvement in organ failures prior to liver transplant reflected by improved prognostic scores improve patient outcomes [11]. The findings in this series is also similar to a study in 2018 by Niu., et al. which showed that DPMAS effectively improved coagulation function, and inflammatory response in ACLF patients [15]. No adverse effects from the use of DPMAS were encountered by our patients.
The authors acknowledge the limitations of this case series. The limited number of patients may not reflect the overall benefit of DPMAS. A large multicenter study is suggested to define the additional indications for DPMAS and timing of this modality based on available prognostic score systems. Based on the patients included in this series, there was improvement on the coagulation function, bilirubin and ammonia level, conditions for hepatocellular regeneration, and transplant-free survival after DPMAS.
DPMAS is a promising modality for bridging ALF and end-stage liver disease to transplantation, and in some cases, recovery to compensated state.
Copyright: © 2024 Jose Orlando M Nicolas., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.