Murittige Gopalakrishna Madhura1*, Vijayalakshmi S Kotrashetti2, Veerendra Kumar B3, Puneetha S4, Naveen Krishnamoorthy5, Mukunda R6, Sreelakshmi R Nair7, Shashank S Bhat7, Suma S8, Gouri S Panchannavar9 and Prashanth CS10
1Research Scholar, Department of Oral Pathology and Microbiology, Maratha Mandal’s NGH Institute of Dental Sciences and Research Centre and Reader Associate Professor, Department of Oral Pathology and Microbiology, DAPM R V Dental College Bengaluru, Rajiv Gandhi University of Health Sciences, Karnataka, India
2Professor and Head, Department of Oral and Maxillofacial Pathology and Microbiology, Maratha Mandal’s Nathajirao G Halgekar, Institute of Dental Sciences and Research Centre, Rajiv Gandhi University of Health Sciences, Karnataka, India
3Professor and Head, Department of Oral Pathology and Microbiology, DAPM R V Dental College, Rajiv Gandhi University of Health Sciences, Karnataka, India
4II MDS (Postgraduate), Department of Oral Pathology and Microbiology, DAPM R V Dental College, Rajiv Gandhi University of Health Sciences, Karnataka, India
5General Pathologist, Koushik Laboratory and Clinic, Bengaluru, Karnataka, India
6Intern, DAPM R V Dental College, Rajiv Gandhi University of Health Sciences, Karnataka, India
7BDS Graduates (Alumni), DAPM R V Dental College, Rajiv Gandhi University of Health Sciences, Karnataka, India
8Reader (Associate Professor), Department of Oral Pathology and Microbiology, DAPM R V Dental College, Rajiv Gandhi University of Health Sciences, Karnataka, India
9Senior Lecturer, Department of Oral Pathology and Microbiology, DAPM R V Dental College, Rajiv Gandhi University of Health Sciences, Karnataka, India
10Principal, DAPM R V Dental College, Rajiv Gandhi University of Health Sciences, Karnataka, India
*Corresponding Author:Murittige Gopalakrishna Madhura, Research Scholar, Department of Oral Pathology and Microbiology, Maratha Mandal’s NGH Institute of Dental Sciences and Research Centre and Reader (Associate Professor), Department of Oral Pathology and Microbiology, DAPM R V Dental College Bengaluru, Rajiv Gandhi University of Health Sciences, Karnataka, India.
Received: September 26, 2024; Published: October 23, 2024
Citation: Geetanjeli Sheogobind., et al. “The Consequences of Untreated Amelogenesis Imperfecta Type III in a 19-year-old African American Female: A Case Study". Acta Scientific Dental Sciences 8.11 (2024):46-53.
Oral squamous cell carcinoma (OSCC) is the common type of head and neck cancer, characterized by genetic and epigenetic abnormalities. There are numerous molecules and mechanisms involved in the etiopathogenesis and progression of OSCC. One such mechanism is autophagy that has been considered as a double edged sword in cancer. Autophagy is a well conserved and orchestrated cellular degradation seen in health and disease. The role of autophagy has been studied in cancer for the past two decades. Autophagy is being proposed to be a potential therapeutic target for many types of cancer. Thus, the aim of the present paper is to highlight the available evidence on the therapeutic role of autophagy in cancer in general, along with an emphasis in oral squamous cell carcinoma. The English databases (Google scholar, Pubmed and Scopus) were searched with key words (autophagy, oral squamous cell carcinoma, therapeutic target) in different combinations for the past 30 years. Both original research and review articles have been considered for the current paper. Evidence from the available English literature on autophagic role in oral squamous cell carcinoma (OSCC) has been summarized along with future outlooks for it to be exploited as an adjuvant target for treating oral carcinoma.
Keywords: Autophagy; Cellular Degradation; Oral Squamous Cell Carcinoma; Therapeutic Target, Tumor promoter, Tumor Suppressor
ATG: Autophagy Related Proteins; ATG4D: Autophagy Related 4D Cysteine Peptidase; ATP: Adenosine Triphosphate; CAFs: Cancer-Associated Fibroblasts; CCL2: Chemokine (C-C Motif) Ligand 2; CCL7: Chemokine (C-C Motif) Ligand 7; CMA: Chaperone-Mediated Autophagy; DAB2IP: Disabled-2 Interacting Protein; EGF: Epidermal Growth Factor; EMT: Epithelial Mesenchymal Transition; FOXE1: Forkhead Box E1; HepG2: Hepatoblastoma Cell Line; HIF-1α: Hypoxia Inducible Factor-1alpha; HSC70: Heat Shock Cognate Protein with Molecular Weight 70kDa; IRF2: Interferon Regulatory Factor 2; IL-1β: Interleukin-1beta; IL-33: Interleukin -33; JAK/STAT: Janus Kinase/Signal Transducers and Activators of Transcription; LC3-II: LC3-Phosphatidylethanolamine Conjugate; miRNAs: MicroRibonucleic Acids; NFkB: Nuclear Factor Kappa B; OncTKs: Oncogenic Tyrosine Kinases; OSCC: Oral Squamous cell Carcinoma; PI3K/AKT/mTORC1: phosphoinositide 3 Kinase/Protein Kinase B/Mammalian (or Mechanistic) Target of Rapamycin (mTOR); PDGF: Platelet Derived Growth Factor; Rab9: RAS Related Protein 9; RAB5A: RAS Related Protein 5A; RAS/MAPK: Rat Sarcoma/Mitogen Activated Protein Kinase; RANKL: Receptor Activator of Nuclear Factor kB Ligand; RTKs: Receptor Tyrosine Kinases; STMN1: Stathmin 1; TAMs: Tumor Associated Macrophages; TGF-β: Transforming Growth Factor – Beta; TKs: Tyrosine Kinases; TKIs: Tyrosine Kinase Inhibitors; TME: Tumor Microenvironment; TLR4: Toll-Like Receptor 4; ULK1: Unc-51-Like Kinase 1
Oral squamous cell carcinoma (OSCC) represents the common type of head and neck cancer. Oral cancer comprises of cancer of the lip and other parts of the oral cavity including oropharynx. Oral cancer has been ranked as the 13th most common cancer across the globe.
The estimated global incidence rate for new cases of lip and oral cavity cancers is around 3.5 lakh and deaths at around 1.5 lakh cases in 2020. Further, oral cancer has been reported to be common in older males when compared to females and is also influenced by various socio-economic situations [1]. Around 3,00,000 individuals are affected annually especially in developed countries.
Evidence has shown that OSCC is attributed to genetic instability and epigenetic vulnerability.
The epigenetic susceptibility is on the rise owing to usage of tobacco, alcohol, or exposure to oncogenic viruses, radiation and such other risk factors.
The constant unravelling of the complex molecular mechanisms involved in the etiopathogenesis and progression of oral cancer has paved the way for discovery and application of diverse diagnostic and prognostic biomarkers towards tailored treatment, all these aiming at improving the overall patient survival [2].
Among the various molecules, mechanisms, pathways and the hallmarks involved in the development and progression of human cancer, autophagy has gained much significance for the past two decades.
The present paper aims at highlighting few key aspects of autophagy as an adjuvant therapeutic target in oral cancer.
For the current paper, the search strategy had comprised of several English databases (Google scholar, Pubmed and Scopus) with specific key words (autophagy, oral squamous cell carcinoma, therapeutic target) in different combinations, for the past 10 years.
Autophagy is a highly conserved process of cellular degradation (derived from Greek referred to as “self” and “eating”), conserved from yeasts to mammals [3].
Autophagy helps in maintaining homeostasis by degrading excessive and or long-lived proteins and also the damaged organelles, mediated by lysosomal machinery; this mechanism provides biological material and energy to meet demands of the cells especially during nutrient deprivation [4]. Autophagy is a multistep process well orchestrated by a set of proteins known as autophagy – (ATG-) regulated proteins studied in autophagy – defective yeast mutants [5].
Autophagy is observed in both physiological and pathological conditions. Based on the mode of transport of cellular products to be degraded by the lysosomes, autophagy could be of 3 types-microautophagy, macroautophagy and chaperone-mediated autophagy (CMA).
The term autophagy commonly used refers to macroautophagy if not specified. In macroautophagy, there is formation of a phagophore that contains the senescent cellular organelles or the cytoplasmic products to be degraded (cargo); this phagophore is a double-membraned structure that gradually matures, seals, with the emergence of autophagosome to fuse finally with lysosome for degradation of its contents. Later, the degradation products get recycled through the cellular anabolic processes [6].
In case of microautophagy, there is invagination of the lysosomal membrane that facilitates degradation of cellular contents (cargo) within the lumen of lysosome [7].
CMA involves participation of the chaperone heat shock cognate protein with molecular weight 70kDa (HSC70) that facilitates the delivery of target proteins containing KFERQ/KFERQ-like sequence motifs towards lysosomal lumen for further degradation [8].
In general, ‘Autophagy flux’ refers to formation of autophagosome with secluding of cargo, followed by lysosomal degradation [9].
The two identified signaling pathways that regulate autophagy are canonical and non-canonical and among 41 proteins, ATG5 has been shown to play a pivotal role in both these pathways (ATG- refers to Autophagy regulated gene). Thus, ATG5 qualifies to be a targeted gene in autophagy gene editing assays. Further, ATG5 has been demonstrated to regulate key aspects of the immune system and it also influences apoptosis.
Under normal circumstances, the baseline autophagy is observed during starvation or it could be induced by oxidative stress, immunological, ischemic and or toxic insults. The cytoprotective function of autophagy is seen to be jeopardized in several diseases and conditions. Association of autophagy has been shown in neurodegenerative diseases, cardiomyopathy, fatty liver, Type II diabetes mellitus, tumor formation, Crohn’s disease, presentation of antigens and defense against intracellular pathogens [10-15].
When compared to normal cells, the cancer cells exhibit greater dependency on autophagy owing to the changes at the cellular level and in energy demands. The molecular oxygen, carbon and nitrogen are the major ingredients for the highly proliferative cancer cells which depend on aerobic glycolysis and glutaminolysis. When there is restriction for the cancer cell to use glucose and glutamine, the cancer cell begins to promote autophagy mediated stress response.
Inhibition of autophagy could be an effective therapeutic strategy especially in advanced cancer. A profound understanding of the intricacies of autophagy modulation may help in designing clinical trial strategies and novel therapeutic interventions [16].
The family of tyrosine kinases (TKs) play important role in oncogenesis. The activation of oncogenic tyrosine kinases (OncTKs) and receptor tyrosine kinases (RTKs) result in autophagy modulation via PI3K/AKT/mTORC1 and RAS/MAPK signaling pathways. Although TKs and RTKs have been targeted in cancer treatment, drug resistance and disease relapse continue to be the major limitations with tyrosine kinase inhibitors (TKIs). Thus, use of TKIs and autophagy modulation are in the ongoing clinical trials for cancer therapy [17].
There are reported studies and clinical trials where anticancer drugs have been combined with modulators of autophagy [18-35]. (Tables 1 and 2).
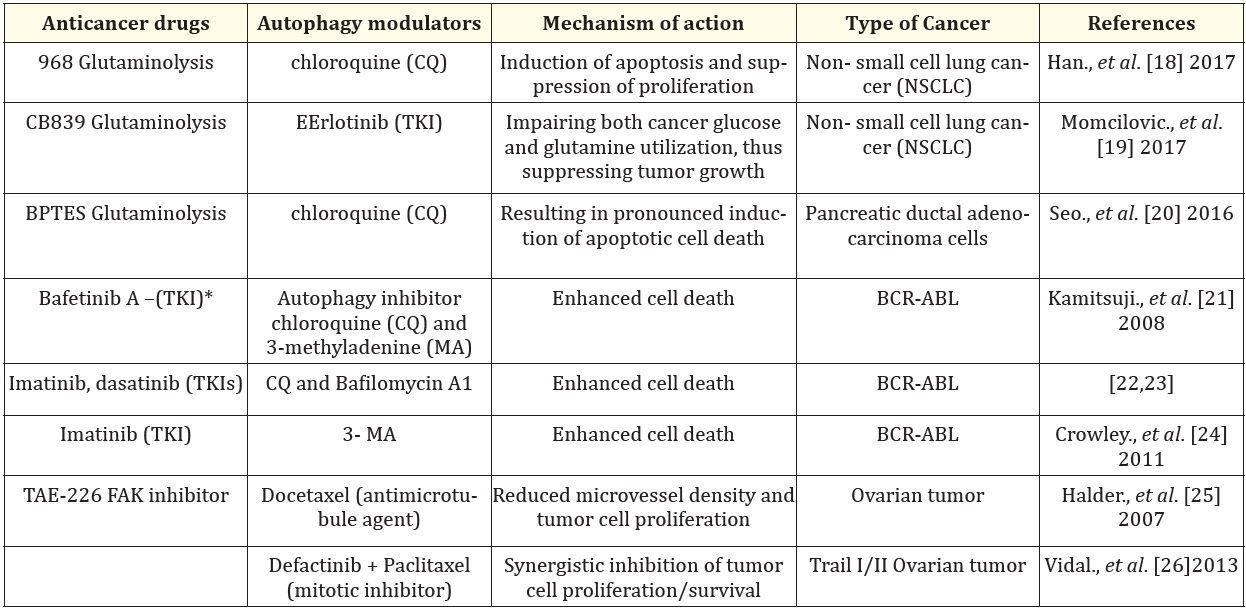
Table 1: Anticancer drugs combined with autophagy modulators in preclinical studies.
*TKI: Tyrosine Kinase Inhibitor
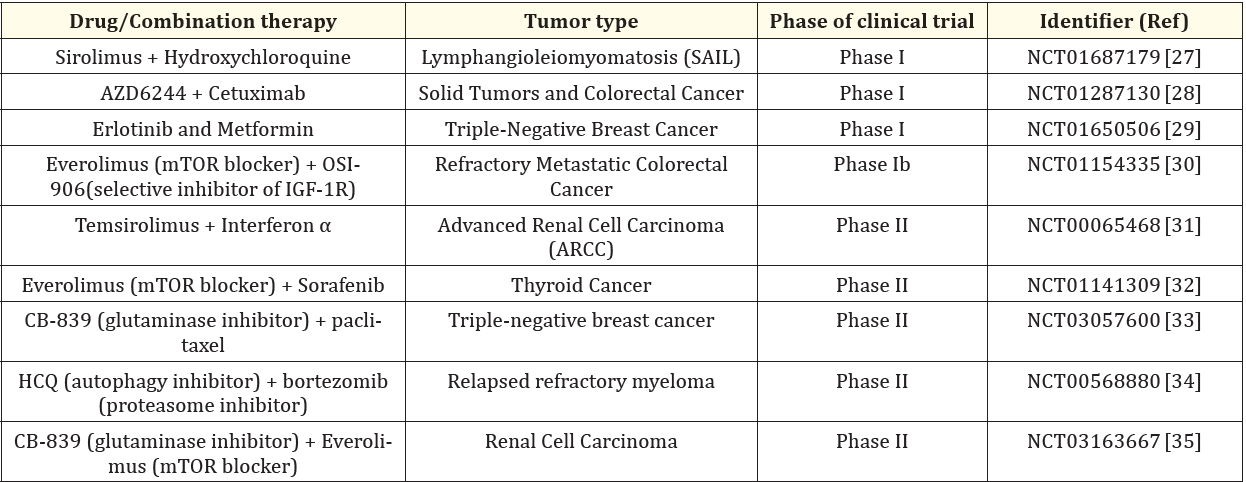
Table 2: Clinical trials where autophagy modulators have been studied in combination with anticancer drugs in cancer therapy.
Warburg had proposed the concept of single compartment model wherein the cancer cells in tumor microenvironment maintain their energy requirements through aerobic glycolysis. Further, mitochondrial dysfunction results in metabolic reprogramming irrespective of the presence of oxygen resulting in lactagenesis. The so produced lactic acid in the extracellular matrix makes the tumor environment more acidic and favors growth of cancer cells. Thus, lactate shuttle has been marked as an essential component of cancer treatment [36].
However, in the tumor microenvironment the aerobic glycolysis occurring in the nearby stromal cells and or the cancer-associated fibroblasts (CAFs) further facilitating the tumor growth has been termed the modified concept or Reverse Warburg effect. It is a two-compartment model with metabolic symbiosis between stromal cells/CAFs and the cancer cells [37].
Glucose is an important metabolite that is being used by the cancer cells for generating energy (ATP, Adenosine triphosphate). This energy leads to biomass formation, helps to regulate the redox state and many other such processes in the TME.
The catabolism of glucose into lactate has been reported to occur profoundly in the cancer cells, especially in CAFs with overexpression of lactate transporters. The oxidative stress induces interplay of various transcriptional activators (HIF-1α), cell cycle regulators (Caveolin-1), tumor promoters and suppressors (TGF-β) in the TME, bringing out autophagy of stromal cells and transformation of cells into CAFs [38,39].
Points of clinical relevance: [37-39].
Autophagy in OSCC tumor microenvironment
The tumor microenvironment of oral carcinoma comprises of stromal cancer-associated fibroblasts (CAFs) as chief non-immune cells and infiltration of immune cells.
The normal fibroblasts get transformed into CAFs through autocrine stimulation via oral carcinoma cell mediated nuclear factor kB (NFkB) dependent mechanism. OSCC cells release interleukin-1β (IL-1β) that activates NFkB in fibroblasts. Around tumor cells, the raised levels of platelet derived growth factor (PDGF), IL-1β or hypoxia would activate Janus Kinase (JAK)/STAT and NFkB pathways in CAFs; this may induce the release of chemokines (CCL2, and CCL7) and growth factors (EGF, Epidermal Growth Factor) or inhibit the release of few miRNAs (Eg. miR34a-5p). All these mediators would enhance proliferation of tumor cells and also facilitate epithelial mesenchymal transition (EMT) of cancer cells.
In the initial stages of oral carcinoma, the long non-coding RNA FLJ22447 inhibits autophagy in CAFs, hindering the autophagic degradation of IL33. The raised levels of IL33 liberated from CAFs induce proliferation of oral carcinoma cells. These carcinoma cells enhance autophagy that would release IL1β that in turn would accelerate autophagy in CAFs mediated by NFkB dependent mechanism. The NFkB mediated autophagy in CAFs release chemokines, CCL7 that acts on OSCC cells, encouraging EMT and impede autophagy.
During advanced stage of oral carcinoma, autophagy decreases Toll-like receptor 4 (TLR4)-dependent EMT. Thus, reduction of autophagy by CCL7 would facilitate TLR4-dependent EMT. Finally, the receptor activator of nuclear factor kB ligand (RANKL) released by OSCC cells induces autophagy in oral carcinoma cells and also promotes transformation of tumor associated macrophages (TAMs) into osteoclasts, fostering metastasis [40].
The MicroRNAs (miRNAs) represent single stranded, small non-coding portion of human genome and are 19-26 nucleotide long. These miRNAs regulate <30% of human genome at posttranscriptional and translational levels. Single miRNA modulates expression of multiple target genes; on the contrary multiple miRNAs may control single target gene expression. The miRNAs regulate cellular proliferation, differentiation, apoptosis, autophagy in health and disease including cancer. Some miRNAs may exhibit oncogenic role while others act as tumor suppressors [41].
Many miRNAs regulate the expression of autophagy related proteins and genes in cancer. These miRNAs influence the various phenomena such as cancer cell proliferation, metabolism, oxidative stress, apoptosis, invasion, metastasis and response to cancer treatment. A lot of autophagy-influencing miRNAs have been identified as diagnostic and prognostic biomarkers in cancer research.42 Few such reported examples have been mentioned here
As the effects of specific miRNA regulated autophagy gene remains one –sided, multidimensional studies with extensive public databases looking at various potential miRNA associated autophagy genes may help in understanding the complex nature of these intricate mechanisms.
Autophagy is characterized by the formation of double membrane vesicles referred to as autophagosomes. These autophagosomes seclude the cargo to be degraded and fuse with lysosomes to bring about lysosomal breakdown of this cargo. Autophagy plays a binary role on oral carcinoma. The knowledge on autophagy and its complex molecular interconnectedness with cellular metabolism is becoming rapidly expanding owing to the emerging preclinical and clinical studies.
In exploiting as a cancer therapeutic adjunct, the cargo receptors and selective autophagy have been the major focus that could pave the way towards newer therapeutic approaches.
Known to act as a double edged-sword, the role of autophagy in the treatment of cancer and in specific oral cancer requires multicentric comprehensive clinical studies with follow up.
The authors would like to acknowledge the valuable inputs given by Dr. Ramakant Nayak, Principal, Maratha Mandal’s NGH Institute of Dental Sciences and Research Centre Belagavi, India and Dr. KS Gopinath, Consultant Surgical Oncologist Bengaluru, India.
No financial interest or any conflict of interest exists.
Copyright: © 2024 Murittige Gopalakrishna Madhura.,et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.