Debashish Nasker1, Shihabul Islam5, Abul Kalam Azad1, Ali Asgor Moral2, Shamsul Alam1, Helal Uddin3, Khaleda Akter1, Ferdousi Begum1, Arup Kumar Saha4, Sabiha Kamal5, Tabassum Jabin5 and Pranab Karmaker5*
1Department of Conservative Dentistry and Endodontics, Bangabandhu Sheikh Mujib Medical University Dhaka, Bangladesh
2Faculty of Dentistry, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
3Department of Orthodontics, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
4Department of Dental Public Health, City Dental College and Hospital, Dhaka, Bangladesh
5Bangladesh Reference Institute for Chemical Measurements (BRICM), Dhaka, Bangladesh
*Corresponding Author: Pranab Karmaker, Bangladesh Reference Institute for Chemical Measurements (BRiCM), Dhaka, Bangladesh.
Received: September 23, 2024; Published: October 14, 2024
Citation: Pranab Karmaker., et al. “A Comparative Analysis on Apexification of Immature Permanent Teeth with Open Apices by Mineral Trioxide Aggregate and Calcium Hydroxide". Acta Scientific Dental Sciences 8.11 (2024):22-30.
Background: Apexification is a procedure used to treat immature permanent teeth when root development stops due to the death of the pulp necrosis. Calcium hydroxide Ca (OH)2 and mineral trioxide aggregate (MTA) are commonly used for inducing apexification at an open tooth root. The purpose of this study was to compare the efficacy of Ca (OH)2 and MTA for apexification of immature permanent teeth.
Methods: This study was conducted on 32 non-vital immature permanent teeth dividing into two groups: Ca (OH)2 treated (control) and MTA treated (experimental). The success rate was determined based upon the time duration required for apical barrier formation. Standard endodontic procedures were followed and an apical barrier of 3 to 5 mm was created by using MTA after and Ca (OH)2. The radiographic evaluation was carried out at a follow up period of 3, 6 and 12 months interval and this evaluation was done to determine the complete or incomplete of apical closure and size of the apical lesion. Chi-square test was used to determine the results statistically significant.
Results: The results of our current study suggested that the lesion size was measured as 2.52 ± 0.54 mm and 2.19 ± 0.24 mm treated by Ca (OH)2 and MTA respectively after 12 months observation period. In case of periapical healing, a significant higher barrier formation (P value 0.040) was observed in 5 teeth (31.2%) due to use of MTA rather than Ca (OH)2 used after 12 months observation period.
Conclusion: So, it can be concluded that induction of apical closure by MTA showed more effective than of conventional Ca (OH)2 method.
Keywords: Apexification; Apical Closure; Calcium Hydroxide; Immature Permanent Teeth; Mineral Trioxide Aggregate
Apexification is a treatment method for immature permanent teeth with halted root growth due to pulp necrosis, commonly using calcium hydroxide (Ca (OH)2) or mineral trioxide aggregate (MTA). This study compared the efficacy of these materials on 32 non-vital immature permanent teeth, measuring apical barrier formation over 12 months’ time interval. Results showed that MTA-treated teeth had a significantly better efficacy compared to Ca (OH)2 treated teeth. So, MTA is more effective for inducing apical closure than Ca (OH)2.
Dental injuries are among the most prevalent health complications in children aged six to sixteen. The primary complication of dental trauma is pulp necrosis, which results from a lack of root maturation and apical closure [1]. This endodontic treatment of young non-vital teeth with necrotic pulp and an open apex entails inducing apical closure by apexification methods to create favorable conditions for traditional root canal filling [2]. Apexification is a treatment for immature permanent teeth that have stopped growing and developing due to pulp necrosis. Calcium hydroxide Ca (OH)2 and mineral trioxide aggregate (MTA) are frequently utilized to stimulate apexification at an open tooth root [3].
For the last few decades, calcium hydroxide (Ca (OH)2) has been the preferred material. Hermann first presented it in 1920. Since Kaiser and Frank originally described the use of Ca (OH)2 in apexification in 1960, it has been utilized to stimulate the creation of an apical hard tissue barrier in immature open apices with remarkable success [4]. The duration required for full apexification using Ca (OH)2 has been observed to vary, ranging from 5 to 20 months or 12.9 months on average, due to its diverse biological effects. Numerous experts have proven its effectiveness through numerous long-term studies, with success rates varying from 74% to 100%. Moreover, Ca (OH)2 is reasonably priced, easily accessible, and simple to utilize and it is still a commonly performed clinical technique [5,6].
In dentistry, the search for better materials is a never-ending journey. Many materials have been developed, examined, and refined in order to provide optimal advantages for superior clinical performance. Dr. Mahmoud Torabinejad introduced mineral trioxide aggregate (MTA) to the area of dentistry in 1993 at Loma Linda University in California, USA. Because of its remarkable and enhanced physical and chemical qualities, MTA has been employed in endodontics [7-10]. The apexification process has undergone new developments, with MTA serving as a main mono block. Apatite-like interfacial deposits emerge during the maturity of MTA, filling in the gap caused by the material shrinkage phase that follows its placement and enhancing MTA's frictional resistance to root canal walls. Because of its alkaline pH, MTA has better biocompatibility and is less cytotoxic. Its high sealing ability, low solubility, regeneration powers, and antimicrobial qualities have garnered a lot of interest [11]. Its formulation's inclusion of calcium and phosphate ions aids in drawing osteoblastic cells and creates an atmosphere that is ideal for cementum deposition, or osteo-cementum [12].
MTA has been recommended as a root-end filling material for endodontic therapy because it exhibits lower levels of inflammation and less cytotoxicity than other retro-filling materials. It also promotes hard tissue induction in the periodontal tissues, stimulates the formation of dentin bridges next to the dental pulp, and has superior sealing capabilities thanks to improved marginal adaptation. Moisture does not impair MTA's ability to seal endodontic and periodontal spaces, making it one of the best materials for sealing both iatrogenic and pathogenic communication [13]. The benefit of MTA is the quick obturation of juvenile roots with broad open apices. Additionally, MTA has been utilized in the healing of root and furcal perforations, pulpotomy, internal and external root resorption, and direct pulp capping [14-16].
Many investigations were conducted to assess MTA's and Ca (OH)2's respective abilities to induce apexification. Therefore, the purpose of this study was to compare the radiographic effectiveness of MTA and the conventionally employed Ca (OH)2 in stimulating the production of immature roots in injured young permanent anterior teeth.
The present study involved the selection of twenty-three healthy individuals from the outpatient department of conservative dentistry and endodontics at Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh, who displayed 32 teeth with non-vital permanent teeth with open apices. The patients were between the ages of 8 and 20. As a result, before the study was entered, all inclusion and exclusion criteria were reviewed following radiological evaluation. The 32 teeth were randomly divided into two groups using a randomized procedure in order to reduce allocation bias. The Group-I (Apical closure induction was done by Ca (OH)2) considered as control group and the Group-II (Apical closure induction was done by MTA) considered as experimental. Following the patient's or patient's guardian's signed informed consent, data was gathered. Each case included a radiograph as well as a thorough medical and dental history, as well as the patient's symptoms and clinical indicators. The same dental radiologist reviewed the radiographs and noted them in the data collecting form. appropriate patient and/or parent counseling regarding the course of treatment, its advantages, and its disadvantages. cleaning the teeth to remove calculus and plaque.
The essential instruments and chemicals used in this study were as follows: Calcium hydroxide Ca (OH)2, Mineral trioxide aggregate (MTA), Sodium hypochlorite, Glycerin, Zinc oxide, Normal saline, Hand gloves, Face masks, X-ray machine, Cotton roll, Saliva ejector, Gutta percha points, Glass ionomer cement and so on. All the chemicals used in this experiment were of analytical grade and available in the market.
The operation field was cleaned and the instruments were properly sterilized on the first visit. In each case, aseptic handling of hand gloves and face masks was employed. Using a bisecting angle approach, an intraoral radiograph was acquired prior to surgery. Saliva extractor and cotton roll were used to isolate the teeth. Diamond round and fissure burs were used to create a straight-line access cavity while adhering to normal procedure. Radiography was used to determine working length. Hedstrom files were used with caution to debride the canal, and 2.5% sodium hypochlorite was used for extensive irrigation. A blunt, sterile paper point was used to dry the canal. Using lentulospiral, Ca (OH)2 powder and glycerin were inserted into the canal in accordance with the Faval., et al. (1999) technique [17].
The access cavity was temporarily filled using zinc oxide eugenol cement and cotton pellets. The patient was told to come back in a week. Cotton and a temporary filling were taken out of the access cavity on the second appointment. To fully eliminate the Ca (OH)2 paste, the canal was irrigated with 2.5% sodium hypochlorite and then regular saline. Once more, the canal walls were rasped to clear any debris, and the canal was dried with a blunt paper point. Ca (OH)2 paste was poured into the canal using a lentulospiral that was the appropriate working length. Radiological verification verified that Ca (OH)2 was positioned correctly. Ca (OH)2 paste that was too much was taken out of the pulp chamber. The access cavity was temporarily filled using zinc oxide eugenol cement and cotton pellets.
After a month, the patient was brought back for their final appointment, during which the canal was closed, watered, and dried. In order to verify the calcific barrier, a gutta percha point was inserted into the canal and radiographic confirmation was obtained. The remaining portion of the canal was sealed using gutta percha and zinc oxide eugenol sealer using a vertical compaction technique if the calcific barrier (2-3 mm) had formed. Glass ionomer cement was used to close off the access cavity. To verify the three-dimensional obturation, an intraoral periapical radiograph was acquired after the procedure.
At the initial visit, the operation field was disinfected and the instruments were properly sterilized. Hand gloves and face masks were utilized in all cases in an aseptic manner. A preoperative intraoral radiograph was obtained using the bisecting angle approach. Teeth were isolated using cotton rolls and a saliva ejector. A straight-line access cavity was made using a diamond round and fissure bur in accordance with normal practice. The working length was determined by a radiograph. Canal debridement was performed carefully using a Hedstrom file, followed by extensive irrigation with 2.5% sodium hypochlorite and normal saline.
The canal was dried with a blunt, sterile paper tip. Ca (OH)2 powder mixed with glycerin was introduced into the canal via lentulospiral, following the methodology reported by Faval., et al. (1999) [17]. Temporary filling of the access cavity was done with cotton pellet and Zinc oxide eugenol cement. Patient was advised to revisit after one week. At second visit, temporary filling and cotton were removed from the access cavity. The canal was irrigated with 2.5% sodium hypochlorite followed by normal saline to remove all the Ca (OH)2 paste properly. Canal was dried with blunt sterile paper point and checked for any exudates. If any exudates was noticed, the Ca (OH)2 dressing was repeated for further one week. A custom made plugger was prepared by heating and rolling Pro Taper gutta percha to condense the MTA at the apex. An intraoral periapical radiograph was taken to confirm that the plugger was at least 3-5 mm short of the apex. Following the procedure outlined by Sharma., et al. 2008) [18], the ProRoot MTA (Dentsply) powder was given to the canal using lentulospiral after being blended to a thick, creamy consistency with distilled water (3:1). The plugger was then placed inside the canal, condensing the MTA to a minimum thickness of three to five millimeters up to the apex. Radiological confirmation of the MTA's correct placement was obtained. The pulp chamber was filled with a moist cotton pellet, and the access cavity was sealed using zinc oxide eugenol cement. The patient was instructed to come back in a day. Cotton and temporary filling were taken out of the access cavity on the last appointment. Using a condenser, the hardness of MTA was assessed. Using a vertical compaction technique, gutta percha and zinc oxide eugenol sealer were used to seal the remaining portion of the canal. The glass ionomer cement was used to close the access. To verify the three-dimensional obturation, an intraoral periapical radiograph was acquired after the procedure.
Following surgery, the patients were instructed to keep their mouths clean and refrain from using any antibiotics or analgesics if they had only little discomfort throughout the follow-up period. After three, six, and twelve months, the patients were also asked back for clinical and radiological assessments.
The radiographs were evaluated by the same dental radiologist using a magnifying glass while they were blind to the treatment record. The radiological evaluations listed below were carried out. Apical shut-off: It was observed if the thin layer of apical bridge covering the MTA or Ca (OH)2 barrier was complete or imperfect. periapical healing was categorized as Whole healing: The periapical lesion fully resolves, not fully healed: significant (more than 50%) decrease in the periapical lesion's diameter, Inadequate healing: The width of the periapical lesion does not decrease or grows. Lastly, a millimetric ruler was used to measure the lesion's diameter.
The study protocol was approved and ethical clearance was given by the Institutional Review Board (IRB) of BSMMU (Reference no: BSMMU/2013/4605). The purposes and procedures of this study were well informed to all of the participants or their guardians and written informed consent was taken from all study participants before inclusion.
This study's statistical analysis was completed with the appropriate hardware and software. Each set of data was presented as mean plus standard deviation (Mean ± SD). Using the Chi-square test and SPSS-20 (Version 27.0. Armonk, NY: IBM Corp.2020) software, the degree of significance was determined. P values less than 0.05 were considered significant at the level of significance after the significance test was computed. At the 5%, 1%, and 0.1% levels, where P∗ < 0.05, P∗ < 0.01, and P∗∗∗ < 0.001, respectively, were the established significant values. Microsoft Excel 2013 was used to exhibit the study's statistics and graphics, and Microsoft PowerPoint 2013 was utilized to assess the figures. To avoid computational error, the zero (0) cells were adjusted by adding 1 in each cell.
In the present study, size of the periapical lesion of the study teeth in both groups (Control and experimental) were evaluated at baseline, 3-, 6- and 12-months interval after completion of the treatment. The results of size of the periapical lesion were showed in Table 1. At baseline, the mean periapical lesion size in MTA (Experimental) and Ca (OH)2 (Control) treated teeth were 5.37 ± 1.41 mm and 3.75 ± 0.86 mm respectively, which was statistically significant at 0.1% level (P value: <0.001) (Table 1 and Figure 1). The results of this study revealed that in both groups, lesion size was decreased after the time elapse. At 3 months follow up period, the average lesion size was seen as 3.38 ± 0.81 mm and 4.56 ± 1.36 mm in control group and experimental group which showed significant results at 1% level (P value: 0.005) (Table 1, Figure 1). Moreover, a significant (at 1% level, P value: 0.001) higher lesion size appeared in experimental group as 3.81 ± 1.11mm than in control group as 2.63 ± 0.62 mm at 6 months follow up interval (Table 1, Figure 1). Finally, at 12 months follow up period, the mean lesion size was reduced to 2.19 ± 0.24 mm and 2.52 ± 0.54 mm in MTA and Ca (OH)2 treated teeth, respectively and again the differences between two groups were statistically significant at 5% level (Table 1, Figure 1).
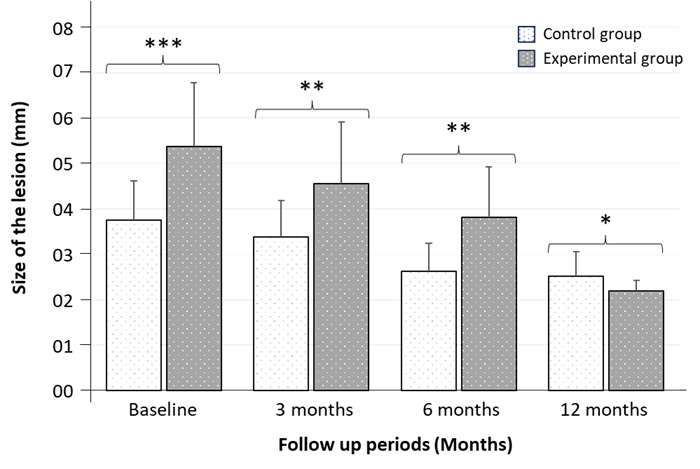
Figure 1: A comparative analysis of lesion size (mm) between Control group and Experimental group after 3-, 6- and 12-months observation period. Data are expressed as Mean ± SD (n = 32) and significance was set at 5% level: P < 0.05 (*), 1% level: P < 0.01 (**), and 0.1% level: P < 0.001 (***) compared to control.
Table 1 shows the comparison of the size of the lesion (mm) between Ca (OH)2 and MTA treated groups following each observation period. It was observed that size of the lesion gradually decreased after elapse of time. However, MTA is more effective in reducing the lesion size than that of Ca (OH)2. The differences between two groups were statistically significant at baseline, 3-, 6- and 12-months observation period.
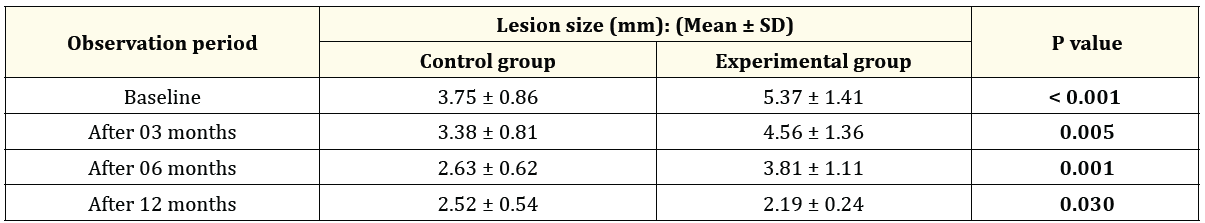
Table 1: A comparative analysis of lesion size between Control and Experimental groups (n = 32).
The size of the periapical lesion of the study teeth in both groups (Control and experimental) were evaluated at baseline, 3-, 6- and 12-months interval after completion of the treatment. The results of periapical healing were showed in table 2. The results of the current study found that, all of the teeth in both groups (Control and Experimental) showed unsatisfactory periapical healing and no complete or incomplete periapical healing was found at baseline observation period (Table 2).
However, periapical healing advanced in both groups. After 3 months of follow up period, about 37.5% teeth of control group showed incomplete periapical healing and 62.5% showed unsatisfactory periapical healing where in experimental group about 75% teeth showed incomplete periapical healing and only 25% showed unsatisfactory periapical healing and the result was statistically significant (P value: 0.030) (Table 2).
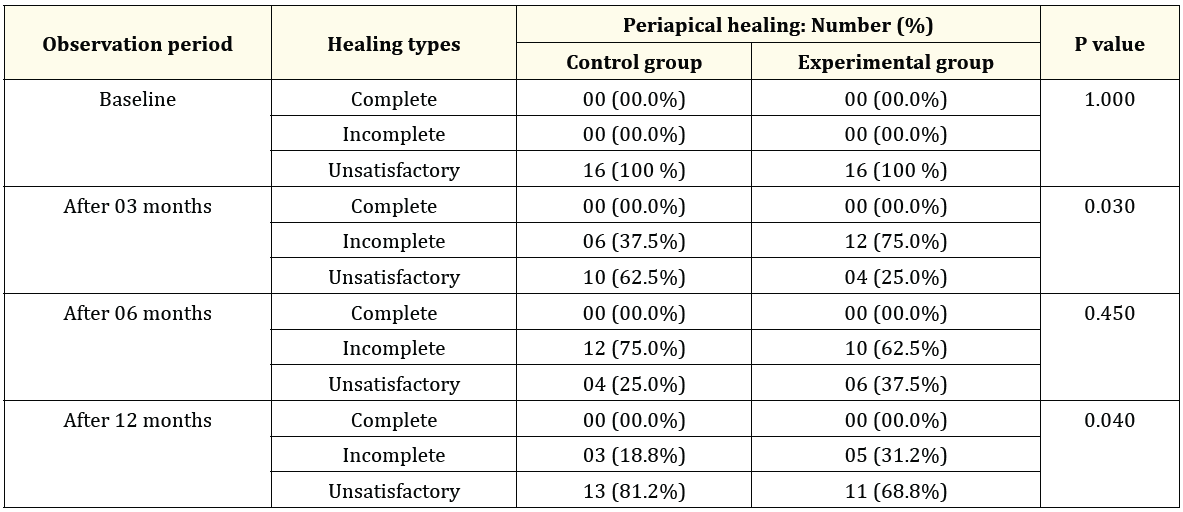
Table 2: A comparative analysis of periapical healing between Control and Experimental groups (n = 32).
Furthermore, at 6 months observation period, about 75% teeth of control group showed incomplete healing and only 25% teeth showed unsatisfactory periapical healing where 62.5% teeth of experimental group showed incomplete healing and 37.5% teeth showed unsatisfactory periapical healing. But the results are not statistically significant at any of the three significance levels (Table 2). Moreover, after 12 months follow up period, about 68.8% of MTA treated teeth (Experimental group) and 81.2% of Ca (OH)2 treated teeth (Control) showed incomplete periapical healing and again the differences between two groups were statistically significant (P value: 0.040) (Table 2).
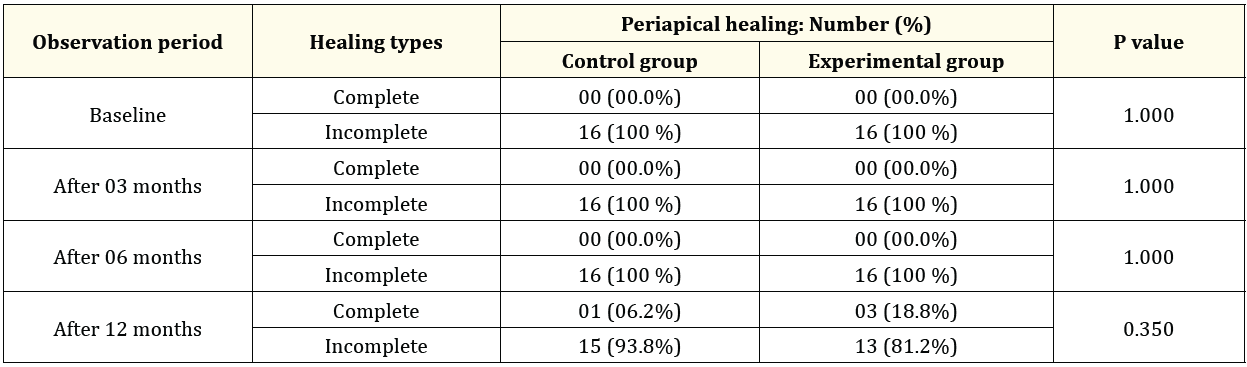
Table 3: A comparative analysis of periapical healing between Control and Experimental groups (n = 32).
The comparison (Shown in Table 2) of the periapical healing between Ca (OH)2 and MTA groups observed that at baseline all cases showed unsatisfactory periapical healing in both groups and after 3 and 12 months follow up, the differences between two groups were statistically significant but after 6 months follow up, the differences between two groups were not statistically significant.
The size of the periapical lesion of the study teeth in both groups (Control and experimental) were evaluated at baseline, 3-, 6- and 12-months interval after completion of the treatment. The results of periapical healing were showed in table 3 and figure 2. in assessing apical closure, at baseline, 3 and 6 months follow up period, all teeth showed incomplete apical closure in both control and experimental groups. But no teeth showed complete apical closure in both groups. Furthermore, after 12 months follow up period, 3 out of 16 (18.8%) of MTA treated teeth (experimental teeth) and 1 out of 16 (6.2%) of Ca (OH)2 treated teeth (control teeth) showed complete apical closure where 13 out of 16 (81.2%) of MTA treated teeth and 15 out of 16 (93.8%) of Ca (OH)2 treated teeth showed incomplete apical closure. The differences between two groups were not statistically significant (P value: 0.350) (Table 3). The comparison shown in Table 3 revealed that the apical closure between Ca (OH)2 and MTA groups followed each observation period. It was observed that at baseline, 3 and 6 months follow up showed incomplete apical closure in both groups. But after 12 months follow up the differences between two groups were not statistically significant. The radiographs shown in figure 2 indicated that the teeth treated with MTA (experimental group) showed comparatively better induction of apical closure than the teeth treated with Ca (OH)2 (control group) after 12 months observation period.
(Figure 2)
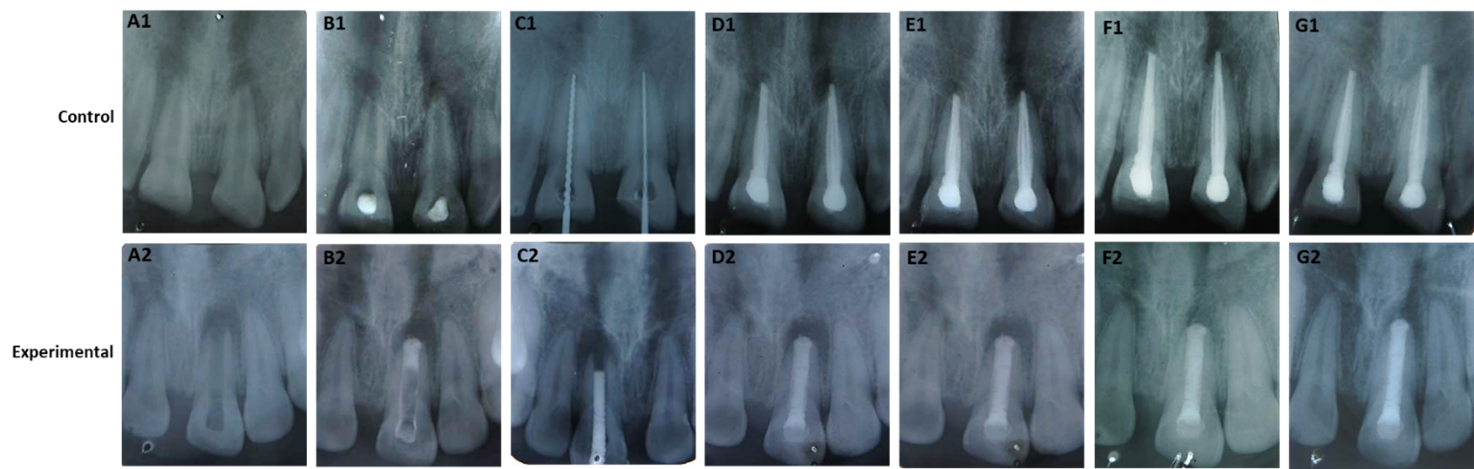
Figure 2: The radiographs of apical closure induction by Ca (OH)2 (control) and MTA (experimental). The radiographs (A1-G1) indicated the teeth treated by Ca (OH)2 and the radiographs (A2-G2) indicated the teeth treated by MTA. In control group: (A1 = initial X-ray; B1 = after placement of Ca (OH)2paste; C1 = 2 mm apical barrier formation; D1 = obturation by vertical compaction; E1 = follow up after 3 months; F1 = follow up after 6 months; G1 = follow up after 12 months). In experimental group: (A2 = initial X-ray; B2 = after placement of MTA paste; C2 = trial of custom made plugger; D2 = obturation by vertical compaction; E2 = follow up after 3 months; F2 = follow up after 6 months; G2 = follow up after 12 months).
In this present study, the induction of apical closure by MTA and Ca (OH)2 were verified in vivo. It was found that one tooth (6.2%) of Ca (OH)2 and 3 teeth (18.8%) of MTA showed apical closure within 12 months following the formation of apical barrier and root canal obturation. Regarding induction of apical closure using MTA, Moore A., et al. (2011) reported that 6 months to 24 months is necessary for complete development of apical closure [19]. Furthermore, Simon S., et al. (2007) have found an apical closure in 26% cases of MTA treated teeth. The results found in this present study were corresponded to that of previous studies. About 18.8% of MTA showed apical closure at 12 months following apical barrier formation and root canal obturation [20].
However, histological investigation by Felippe WT., et al. (2006) indicated that induction of apical hard barrier tissue formation (apical closure) was developed with 100% success rate in an animal study, which differs with this present study [21]. The differences might be due to the evaluation techniques used. In this present study, as the induction was observed radiologically. This might be possible reason of the differences between this present study with that of previous study. Regarding size of the lesion and periapical healing, it was found that lesion size decreased gradually with increasing periodontal healing.
However, according to radiographic observation, it was found that no cases showed complete periapical healing as well as disappearance of the lesion. Again, previous studies have indicated that 2-3 years were required for complete healing [19]. Pace R., et al. (2007) reported that no cases were healed at 12-months but 10 of 11 (91%) was healed at 24 months recall [22]. Zarrabi MH., et al. (2005) found that periapical healing occurs in 100% cases of MTA group while it was only 57% in Ca (OH)2 group [23].
Long-term clinical and radiological studies are therefore required. The biggest diameter of any apical lesion that was present was noted. It was also noted if an apical closure was visible or not. The exact method by which calcium hydroxide induces an apical barrier is still up for debate. The dissociation of calcium ions from Ca (OH)2 is essential for triggering osteoblast mineralization. It functions as a root canal disinfectant and promotes the growth of fibrous and calcified tissue cells (osteo dentin) in the apical portion of the root canal [24].
Numerous publications have shown that calcium hydroxide is effective even when there is an apical lesion [25-27]. According to one theory explaining how MTA causes apical closure, it does so by promoting the synthesis of interleukin (IL-6 and IL-8), which in turn promotes osteo-cementum (the overgrowth of cementum) and bone development, all of which aid in the repair of the periodontal ligament. According to an animal investigation, MTA-induced apexification may also produce an apical hard tissue barrier with a superior consistency than calcium hydroxide [28].
According to clinical investigations, 1 to 3 years following the insertion of MTA apical plugs, 77% to 85% of teeth with open apices fully healed [29,30]. While the precise process of apical closure remains unclear in this investigation, prior research suggests that the apical hard tissue barrier or excessive cementum growth was responsible for forming apical closure. For the apical closure of a permanent necrotic immature tooth, there are also novel and intriguing treatment possibilities on the horizon, like apex regeneration. Two recent areas of research are stem cell regeneration and revascularization by blood clot activation [31,32].
Patients with non-vital permanent teeth with open apices will undoubtedly benefit from quicker and more dependable treatment choices as a result of ongoing research. Throughout the duration of treatment, every patient complied with the guidelines to the letter. The study's control group included participant-induced confounders. Therefore, additional confounding variables are unlikely to have an impact on the study's conclusions.
In conclusion, according to the current study's findings, MTA was more effective than Ca (OH)2 at helping immature permanent teeth with open apices apexify. As a result, it is clear that MTA and Ca (OH)2 are equivalent in terms of the evaluation criteria when utilized as apexification materials, with MTA having the advantage of enabling the quick obturation of immature roots with wide open apices. On the other hand, in order to further support the use of either material in barrier formation and apical healing of open apices of traumatized young permanent anterior teeth, a larger sample size and a longer follow-up clinically and radiographically at regular intervals are required before conclusive conclusions regarding better efficacy among the two materials can be made.
The datasets generated or analyzed during the current study are available from the corresponding author upon reasonable request. This includes all relevant raw data required to reproduce the findings reported in this study, including measurements of apical barrier formation, lesion size, and radiographic evaluations conducted at 3-, 6-, and 12-month intervals. Requests for data access will be considered in accordance with institutional policies and ethical guidelines.
Debashish Nasker and Pranab Karmaker: Conceptualized and designed the study, supervised the research process, and contributed to the writing and revision of the manuscript. Md. Ali Asgor Moral and Md. Shamsul Alam: Conducted the experimental procedures and collected the data. Helal Uddin and Khaleda Akter: Assisted in data collection and performed radiographic evaluations. Ferdousi Begum and Arup Kumar Saha: Analyzed the data and conducted the statistical analysis. Md. Shihabul Islam and Tabassum Jabin: Analyzed the data, conducted the statistical analysis and assisted in data interpretation and manuscript preparation. Pranab Karmaker: Provided overall guidance, coordinated the research activities, and critically reviewed and approved the final manuscript for submission. All authors read and approved the final manuscript.
Copyright: © 2024 Pranab Karmaker.,et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.