Efsun Somay1*, Erkan Topkan2, Sibel Bascil3, Duriye Ozturk4, Sukran Senyurek5 and Ugur Selek5
1Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Baskent University, Ankara, Turkey
2Department of Radiation Oncology, Faculty of Medicine, Baskent University, Adana, Turkey
3Department of Periodontology, Faculty of Dentistry, Baskent University, Ankara, Turkey
4Department of Radiation Oncology, Afyonkarahisar Health and Science University, Afyonkarahisar, Turkey
5Department of Radiation Oncology, School of Medicine, Koc University, Istanbul, Turkey
*Corresponding Author: Efsun Somay, Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Baskent University, Ankara, Turkey
Received: September 25, 2024; Published: October 11, 2024
Citation: Efsun Somay., et al. “The Impact of Oral Health and Antibiotics on the Outcomes of Head and Neck Cancer Patients Receiving Anticancer Therapies: Two Interrelated Conditions". Acta Scientific Dental Sciences 8.11 (2024):09-11.
Oral health assumes a pivotal role in the comprehensive care and quality of life for head and neck cancer (HNC) patients. Maintaining optimal oral hygiene and proficient handling of oral complications before, during, and after cancer treatment are imperative. These measures have the potential to notably diminish complication incidence and severity and augment patient comfort treatment outcomes. Poor oral health can exacerbate treatment-related side effects such as mucositis, xerostomia, and infections and potentially lead to treatment delays or interruptions due to reduced patient tolerance, thereby impacting tumor control and survival results. Using antibiotics to prevent or treat infections during cancer therapy in HNC patients is common practice. Nevertheless, the impact of antibiotics on long-term survival outcomes is multifaceted and influenced by a range of factors, such as the specific antibiotic used, the timing of administration, and patient-specific variables. Therefore, this review consolidates recent research examining the effects of antibiotic usage on the microbiome, immune response, and survival outcomes of HNC patients. It also provides insights for optimizing antibiotic usage strategies to enhance outcomes in HNC patients and highlights the significant influence of oral health disease-related outcomes in this patient population.
Keywords: Head and Neck Cancer; Oral Health; Cancer Risk; Survival Outcomes; Antibiotics
HNC: Head and Neck Cancer; HIV: Human Immunodeficiency Virus; HPV: Human Papillomavirus; EBV: Epstein-Barr Virus; OSCC: Oral Squamous Cell Carcinoma; TT: Tumor Tissue; RR: Relative Risks; CI: Confidence Interval; HR: Hazard Ratio
Head and neck cancers (HNC) rank as the sixth most prevalent cancer type globally, including a varied array of malignant tumors originating from the nasal cavity, paranasal sinuses, nasopharynx, oropharynx, hypopharynx, larynx, and oral cavity [1]. Notwithstanding influential progress in diagnostic, therapeutic, and patient care approaches, HNCs continue to be a significant contributor to cancer-related morbidities and mortalities worldwide [2]. The primary risk factors associated with HNCs include tobacco use, betel nut chewing, alcohol consumption, opium use, alcohol-based mouthwash, periodontitis, immunodeficiency due to Human Immunodeficiency Virus (HIV) infection or solid organ transplantation, human papillomavirus (HPV) infection, and Epstein-Barr virus (EBV) infection. Recent research suggests a strong connection between oral health, treatment strategies against oral issues, the risk of HNC, and patient outcomes following cancer treatment [3-6]. Despite being frequently disregarded, this connection exhibits resilience and merits heightened consideration in clinical and investigative contexts.
The oral tissues of patients undergoing oncological treatment for head and neck cancer (HNC) are particularly susceptible to adverse effects, directly impacting oral hygiene [7]. Periodontitis is an inflammatory condition affecting the supporting structures of the teeth, primarily attributed to specific microorganisms or groups thereof. This inflammatory process leads to progressive degradation of the periodontal ligament and alveolar bone, often presenting as forming periodontal pockets, gingival recession, or both [8]. In its advanced stages, periodontitis can result in tooth loss and is correlated with an elevated risk of heart disease, stroke, and diabetes, thereby complicating the treatment of HNC patients [8,9]. Maintaining good oral health is crucial for HNC patients, as infections may worsen treatment-related complex conditions and affect treatment tolerance and response [10]. In the event of a patient being diagnosed with periodontitis during cancer treatment, it is imperative to conduct a thorough assessment, taking into consideration the urgency of dental intervention, the patient's overall treatment regimen, and the patient's immunological status. This is of particular importance since periodontitis commonly necessitates antibiotic therapy, the administration of which holds the potential to compromise the survival prospects of such patients [7]. Hence, proper periodontitis treatment timing and judicious antibiotic selection are imperative for HNC patients, usually necessitating a multidisciplinary approach. In this context, the present review was conducted to underscore the significance of maintaining oral hygiene in HNC patients, the criticalness of periodontitis treatment timing, and the impact of antibiotic usage on survival.
Multiple studies have consistently demonstrated that poor oral health significantly increases the risk of developing various types of HNCs [3,7,11-13]. Moreover, inadequate oral hygiene can result in chronic local and systemic inflammation by disrupting the balance of microorganisms in the oral cavity. Persistent inflammation can produce and release inflammatory cytokines or chemokines, which may stimulate cell proliferation, enhance cellular invasion capacity, activate oncogenes, and promote tumor angiogenesis [14]. Bacteria experiencing dysregulation in their microbiological balance can lead to the overproduction of carcinogenic end products, thereby enhancing the carcinogenic effects of other carcinogens, such as nitrosamines. Additionally, they can metabolize alcohol into genotoxic chemicals, including acetaldehyde, which can induce DNA damage [15]. Moreover, oral bacterial dysbiosis, particularly involving Porphyromonas gingivalis and Fusobacterium nucleatum, has been implicated in oral cancer's onset in human and animal research studies [16]. Recent clinical research and meta-analysis have irrefutably established a compelling association between inadequate oral health and an elevated predisposition to HNCs. A meta-analysis conducted by Ma., et al. encompassing 16 studies with 6,032 oral cavity cancer patients and 7,432 healthy controls, revealed that periodontitis was significantly correlated with a 2.94-fold increase in the risk of oral cavity cancers (p = 0.032). Similarly, Bai and colleagues conducted a meta-analysis of 44 studies, showing a significant association between poor oral hygiene and HNC incidence. The meta-analysis identified mouthwash use, having more than 5 missing teeth, gum bleeding, and periodontal disease as factors associated with an increased risk of oral cavity cancer [3]. Additionally, the association between poor oral hygiene and the incidence of HNC appears to be unrelated to the individual's age, indicating a consistent risk for HNC development across all adult age groups [18]. This observation underscores the importance of maintaining proper oral hygiene practices to mitigate the risk of HNC occurrence across all age groups. Hashim and colleagues performed a pooled analysis of 8,925 head and neck cancer cases and 12,527 controls from 13 studies under the International Head and Neck Cancer Epidemiology Consortium. Their results demonstrated that having fewer than 5 missing teeth (odds ratio [OR] = 0.78), consistent yearly dental visits (OR = 0.82), daily tooth brushing (OR = 0.83), and the absence of periodontal disease (OR = 0.94) were all correlated with a lower risk of developing HNC, providing practical insights that can warn individuals to take care of their oral health [19] (Figure 1).
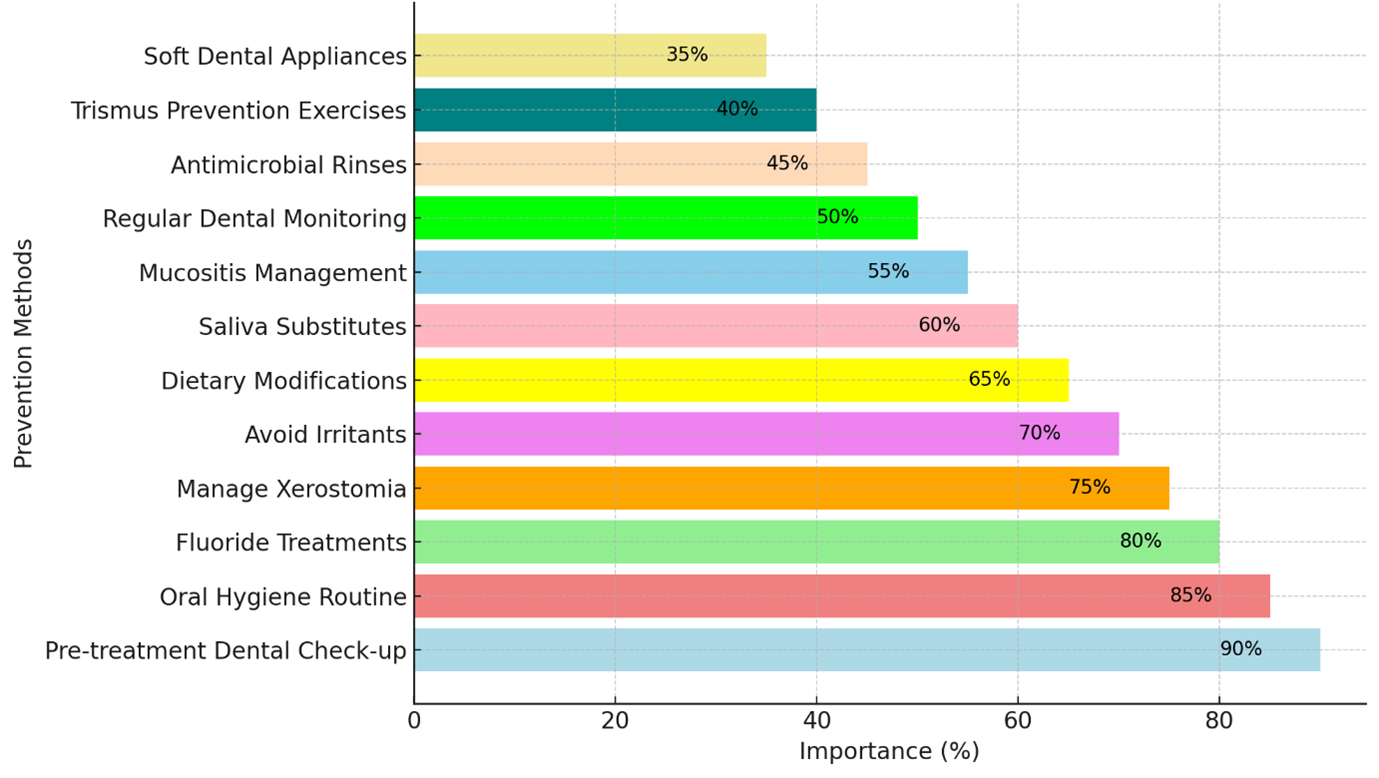
Figure 1: Strategies for enhancing oral health in head and neck cancer patients, ranked by their significance in prevention.
Note: This figure was generated with the help of AI
Although infrequently researched, poor oral health can also detrimentally impact the treatment outcomes of HNC patients, besides being a known cause of such cancers [4-6,20,21]. To elaborate, infectious bacterial byproducts and persistent oral and systemic inflammation may lead to diminished treatment response, potentially affecting both locoregional and distant tumor control and survival rates. The inflammatory responses associated with cancer and bacterial infections play a pivotal role in almost all stages of cancer progression. These stages encompass cellular proliferation, tumor growth, immune system evasion, resistance to apoptotic signals, tumor invasiveness, and metastasis [22]. Furthermore, dysbiotic bacterial proliferation may exacerbate tumor aggressiveness, advancement, and resistance to currently available cancer therapies, including chemotherapy, targeted therapies, immunotherapy, and radiotherapy [23,24]. Three studies have shown that oral dysbiosis can enhance tumor growth in patients with oral cancer [25-27]. In their research, Yung., et al. [25] conducted a comprehensive analysis of the oral microbiota to identify bacterial biomarkers linked to oral squamous cell carcinoma (OSCC). This investigation involved profiling the microbiota in oral mouthwash samples obtained from 51 healthy individuals and 197 OSCC patients at varying stages. The analysis utilized 16S rRNA V3V4 amplicon sequencing and subsequent application of bioinformatics and statistical analyses. The study's findings provided compelling evidence of discernible alterations in oral bacterial communities throughout the progression of OSCC. Specifically, the study revealed an inverse association between the prevalence of Streptococcus mitis, Haemophilus parainfluenzae, and Porphyromonas pasteri and the progression of OSCC. This observation suggests the potential utility of these bacteria as diagnostic markers for OSCC. In a separate pilot study, Yost., et al. [26]. utilized metatranscriptomic analysis to delineate bacterial functional activities in individuals with and without OSCC. The comparison encompassed OSCC-affected regions vis-à-vis adjacent tumor-free areas from OSCC patients and corresponding sites from tumor-free controls in a cross-sectional design. The investigation specifically targeted nonsmoking and HPV-negative OSCC specimens, thus mitigating variability stemming from other high-risk factors for OSCC to a certain extent. The study findings posited that Fusobacteria potentially represents the predominant phylogenetic group accountable for the heightened expression of virulence factors within the oral microbiome of OSCC patients. Furthermore, particular bacterial species, such as Porphyromonas gingivalis, have been associated with increased HNC-related mortalities, as evidenced by Mukherjee and colleagues [27]. In their investigation, Mukherjee., et al. [27] endeavored to examine the bacteriome and mycobiome present in mobile tongue cancers. Specifically, the study sought to discern potential variances in the bacteriome and/or mycobiome between oral tongue cancers and their corresponding normal tissue and to ascertain whether these variances correlated with clinicopathological characteristics. The study results indicated that there was a significant decrease in bacterial diversity and richness as well as fungal richness in tumor tissue (TT) compared to matched non-tumor tissues (NTT, P < 0.006) based on microbiome profile, principal coordinate, and dissimilarity index analyses. The most abundant bacterial species was Firmicutes, and the abundance of Bacteroidetes and Fusobacteria was significantly lower in TT compared to matched NTT (both P ≤ 0.003). Additionally, the tumor group significantly increased the abundance of Streptococcus and 22 other bacterial and 7 fungal genera. The presence of the fungal genus Aspergillus in TT showed a negative correlation with Actinomyces, Prevotella, and Streptococcus and a positive correlation with Aggregatibacter species. The average variances between TT and NTT were lower in patients with advanced T-stage disease than those with early T-stage disease (0.07 vs. 0.21, P = 0.04). Therefore, the study authors showed that the bacteria and fungi present in oral tongue cancers differ from those in healthy oral tissue, and this difference is directly related to the extent of the tumor (T-stage). Additionally, certain bacteria, like Porphyromonas gingivalis, which is linked to periodontitis, are associated with a higher risk of mortality in HNCs (28). For example, Ahn., et al. [28] examined whether periodontal disease assessed by dental examination was associated with oro-digestive cancer mortality, and to directly address microbe-cancer relationships, they also prospectively investigated the association of serum antibody levels to Porphyromonas gingivalis with oro-digestive cancer mortality. The authors reported that periodontitis (moderate or severe) was associated with an increased risk of oro-digestive cancer mortality [relative risks (RR) = 2.28, 95% confidence interval (CI) = 1.17-4.45], with mortality risks increasing with increasing severity of periodontal disease (P for trend = 0.01). Furthermore, they noted that periodontitis-associated mortality was increased for colorectal (RR = 3.58; 95% CI = 1.15-11.16) and possibly pancreatic cancer (RR = 4.56; 95% CI = 0.93-22.29). They also reported that higher serum Porphyromonas gingivalis IgG tended to be associated with increased overall oro-digestive cancer mortality (P for trend = 0.06), demonstrating that Porphyromonas gingivalis is a biomarker of microbe-related mortality risk from oro-digestive cancer, independent of cancer diagnosis.
Figure 2: The effect of radiation on periodontal tissues.
Note: This figure was generated with the help of AI.
Our understanding of the association between oral health and treatment outcomes in patients with HNCs is still nascent. However, the available evidence unequivocally demonstrates that poor oral health is intricately linked to inferior survival outcomes [3-6,21]. In the first investigation on the subject, Farquhar and colleagues assessed oral health indicators' influence on survival within a population-based HNC cohort comprising 1,381 cases and 1,396 age-, sex-, and race-matched controls [4]. Following adjustments for confounding variables, the findings revealed that > 10 routine dental visits over the preceding 10 years correlated with a diminished risk of mortality (hazard ratio (HR) = 0.6), with the most profound impact observed in cases of oral cavity cancer (HR = 0.4). In a recent study by Friemel., et al. it was observed that adherence to a high level of dental care, encompassing regular dental visits, daily teeth cleaning, and floss utilization, correlated with an extended overall survival period (p = 0.001) in individuals with HNC [21]. Conversely, the frequent use of mouthwash, precisely two or more times per day, was associated with an elevated risk of mortality specific to HNC (HR: 2.26). Tasoulas and colleagues [6] recently conducted a pooled examination of data encompassing 2,449 patients with HNC from four studies organized by the International Head and Neck Cancer Epidemiology Consortium. The study's objective was to investigate the influence of oral health on the overall survival outcomes of these patients. The findings indicated a positive correlation between higher numbers of natural teeth (10-19 teeth: relative risk [RR]= 0.81; ≥ 20 teeth: RR= 0.88) and frequent dental visits (> 5 visits: RR= 0.77) with improved overall survival rates. This association was most notable in patients with hypopharyngeal, laryngeal, and otherwise unspecified HNCs regarding natural teeth and patients with oropharyngeal carcinoma concerning dental visits.
Finally, the currently available data indicates a significant correlation between insufficient oral health and heightened susceptibility to HNCs, suboptimal responses to anticancer therapies, diminished tumor control rates, and compromised survival outcomes. Consequently, it is imperative to advocate for comprehensive oral care across the entire population to mitigate the prevalence of HNC and HNC-related mortality. However, attaining this overarching objective necessitates a concerted effort involving HNC professionals and governmental entities.
The majority of head and neck cancers, including those affecting the paranasal sinuses, nasal cavity, oral cavity, pharynx, nasopharynx, oropharynx, hypopharynx, larynx, and salivary glands, are commonly associated with risk factors such as tobacco use, alcohol consumption, betel nut chewing, as well as HPV and EBV infections [30]. Despite substantial advancements in oncological therapy that have led to improved survival rates and reduced toxicity for patients with HNC, maintaining high survival rates for this specific patient population continues to pose a significant challenge [31]. Upon consolidating data from all disease stages, the 2-year mortality rate for HNCs can rise to as high as 47.7%, while the 2-year survival rate hovers around 50% in these patients [32]. Numerous factors may impact the survival outcomes of HNC patients, encompassing tumor stage, nodal stage, metastasis status, tumor grade, resection status, comorbidities, smoking, low hemoglobin levels, poor performance status, immune deficiencies, aggravated local and systemic inflammation, malnutrition, weight loss, and poor therapeutic responses [32-34]. Furthermore, recent data posit that antibiotics may detrimentally affect patients' survival by indirectly influencing the immune system [35,36].
The intersection of cancer and infectious disease management presents a nuanced challenge. An area of particular concern involves the administration of antibiotics to patients with HNC, primarily when these individuals also contend with periodontitis. Periodontitis, a chronic inflammatory condition impacting the gums and supportive structures of the teeth, is prevalent among HNC patients. While antibiotics are frequently prescribed to address bacterial infections associated with periodontitis or other oral cavity infections, emerging research indicates that their usage may inadvertently compromise the survival rates of HNC patients [37].
Patients with HNC often require antibiotic treatment as a preventive measure or to address a wide range of bacterial infections. This practice is necessary due to their increased susceptibility to infections, which results from their compromised immune system compared to healthy individuals [38]. Notably, periodontitis may be as common as approximately 76.9% of HNC patients, often requiring antibiotic treatment and potentially impacting their survival outcomes [7,39]. However, underscoring the complexity of antibiotic use in cancer patients and the necessity for further research to comprehend the potential risks and benefits, there are differing perspectives on the use of antibiotics in cancer patients. Firstly, some suggest that antibiotics exhibit no adverse effects and might even impede the proliferation of cancerous cells by suppressing chronic inflammation when used during chemotherapy or radiation therapy. On the other hand, an alternative viewpoint highlights the potential for antibiotics to induce dysbiosis, which results in an imbalance in the microbial community and can impair the body's immune response to tumors [40,41]. This perspective underscores the potential risks associated with antibiotic use. In support of this latter notion, studies have shown that a healthy gut microbiome increases the effectiveness of immunotherapy in HNC patients, while dysbiosis reduces treatment response [42].
Following their serendipitous discovery, antibiotics have emerged as pivotal agents in treating bacterial diseases. Their therapeutic efficacy primarily stems from their ability to mitigate chronic inflammatory stimuli [43]. Notably, the use of antibiotics in patients with HNC disrupts the microbiome, especially the gut microbiome, which plays a crucial role in immune system regulation. Antibiotic administration can precipitate dysbiosis, characterized by an imbalance in the microbial community that may compromise the immune response to malignancies [44]. The literature reveals that a healthy gut microbiome has been associated with enhanced efficacy of immunotherapy, whereas an imbalance has been correlated with reduced success rates [45]. While the precise mechanisms remain incompletely elucidated, mounting evidence supports the notion that perturbing the intestinal flora with diverse antibiotics may detrimentally impact the gut microbiota. These consequences encompass diminished species diversity, metabolic activity alterations, and antibiotic-resistant bacteria proliferation [46]. Additionally, the emergence of antibiotic-resistant bacteria has been linked to the excessive use or erroneous dosing of antibiotics. Infections arising from these resistant strains present formidable treatment challenges, potentially complicating the clinical management of cancer patients, including those with HNCs, and resulting in heightened levels of morbidity and mortality [47-49]. In an extensive real-world analysis encompassing over 3000 cases, Preissner., et al. observed a marked decrease in the efficacy of immunotherapy after administering antibiotics [36]. This decline was closely associated with antibiotic-induced modifications in the gut microbiome. Furthermore, empirical investigations have revealed a marked association between the use of antibiotics and adverse outcomes in cancer patients. In this regard, Preissner., et al. documented a significant rise in the risk of mortality within a year among individuals who underwent immunotherapy and received antibiotics within ± 3 months, compared to those who did not receive antibiotic therapy [36]. Moreover, a retrospective analysis conducted by Abdelhamid., et al. suggested that patients with head and neck cancer who received antibiotics during their treatment exhibited a lower overall survival rate than those who did not [50].
The precise etiology behind the detrimental outcomes experienced by cancer patients after antibiotic administration remains the subject of active investigation. Nonetheless, it is widely recognized that both perturbations in the microbiome and immunomodulation significantly contribute to these outcomes. Notably, empirical evidence establishes that the substantial and recurrent use of antibiotics, particularly those targeting anaerobic microorganisms such as vancomycin, can disrupt and destabilize the intricate intestinal microbiome, exerting multifaceted repercussions on the tumor–host-microbe interface [51].
Furthermore, it has been empirically evidenced that antibiotics can instigate extensive and prolonged alterations in the host-microbial ecosystem's diversity. Approximately 30% of the bacterial species that make up the gut microbiome might change due to these non-physiological adjustments, which can reduce the essential microbial activities responsible for protecting the host [52]. In addition, it has been shown that antibiotics affect the composition of the human microbiota, altering the effectiveness and possible toxicity of various oncological medications. Ultimately, this interaction may affect the patient's survival [53]. Therefore, while contemplating antibiotic therapy for bacterial infections in HNC patients, making decisions with excessive care is essential.
Periodontitis is an inflammatory condition that impacts the supportive tissues of the teeth, attributing its cause to specific microorganisms or groups. Persistent mild to moderate inflammation leads to the gradual degradation of the periodontal ligament and alveolar bone, often accompanied by the development of periodontal pockets, gingival recession, or both. In severe cases, periodontitis can result in tooth loss and is affiliated with an elevated risk of heart disease, stroke, and diabetes, complicating the treatment of head and neck cancer patients [8,9]. Maintaining optimal oral health is paramount for patients suffering from HNC, as these individuals are particularly susceptible to complications and infections that can compromise treatment efficacy and tolerability [10]. Demonstrating the seriousness of the condition, Heikkila and colleagues have identified an association between periodontitis and heightened overall cancer mortality, with an RR of 1.33 [54]. Regrettably, systemic antibacterial agents, notwithstanding their adverse impact on cancer patients' prognoses, constitute the cornerstone of periodontal therapy, with or without supplementary mechanical interventions [55]. Consequently, this intricate correlation prompts the inquiry of how to address periodontitis in cancer patients while safeguarding their prognoses effectively (Figure 2).
In the context of HNC patients, the early prevention or treatment of periodontitis is of utmost importance to impede its progression, mitigate associated complications, and alleviate its adverse impact on cancer-related clinical outcomes. Essential preventive measures, including routine dental examinations and adherence to sound oral hygiene practices, are imperative. Consequently, addressing periodontitis before initiating cancer treatment holds significant significance. This approach can effectively diminish the probability of oral infections and their related complications, potentially compromising the immune system and impeding the healing process during chemotherapy and/or RT [56]. Moreover, this strategic maneuver may serve to forestall or attenuate the occurrence of neutropenic sepsis in chemotherapy patients, as a notable association exists with infected periodontal tissue in a considerable proportion of cases [57].
In treating periodontitis, it is paramount to prioritize noninvasive interventions aimed at reducing infection and inflammation. When antibiotic therapy is deemed necessary, careful consideration should be given to the patient's immune status and treatment protocol. Previous studies have examined the impact of four commonly prescribed antibiotics-azithromycin, levofloxacin, cefpodoxime, and their combinations—on the commensal microbiome [58]. The findings revealed that the short-term use of these antibiotics, commonly employed to combat bacterial infections, can result in both short- and long-term disruptions and scarring of the microbiome in healthy human volunteers, which may contribute to a sustained elevation of antibiotic resistance within healthy microbiomes [59]. Azithromycin, for example, exerts its efficacy by inhibiting protein synthesis. Thus, it demonstrates a prolonged therapeutic and prophylactic impact persisting for 12 days post-treatment, ostensibly attributed to its protracted half-life within mucosal tissue. Consequently, azithromycin exerts a sustained influence on dysbiosis [60,61].
In situations where antibiotic therapy is deemed necessary for patients undergoing immunotherapy, it is imperative to exercise caution when considering certain antibiotic regimens, particularly those involving azithromycin and its combinations. Research indicates that these specific regimens have been shown to impede the recovery of species richness for a period of two months [36]. Given the known use of antibiotics such as amoxicillin/metronidazole, azithromycin, clindamycin, and clarithromycin in the adjunctive pharmacological management of periodontitis, diligent monitoring and management of post-cancer oral health treatment becomes crucial to mitigate the reliance on antibiotics. This precaution is warranted due to the potential impact of antibiotics on patient survival (Table 1) [61]. Antibiotics can significantly influence patients' gut microbiome, potentially impacting the microbiome's role in the efficacy of RT. Notably, preclinical studies have demonstrated enhanced anti-tumor effects of RT following vancomycin administration, which selectively targets gram-positive bacteria, indicating a complex interplay among RT, patient microbiomes, and antibiotics [62]. In line with these findings, Rühle and colleagues noted a more pronounced reduction in progression-free survival and locoregional control in patients who received two or more antibiotics during (chemo)radiation [63].
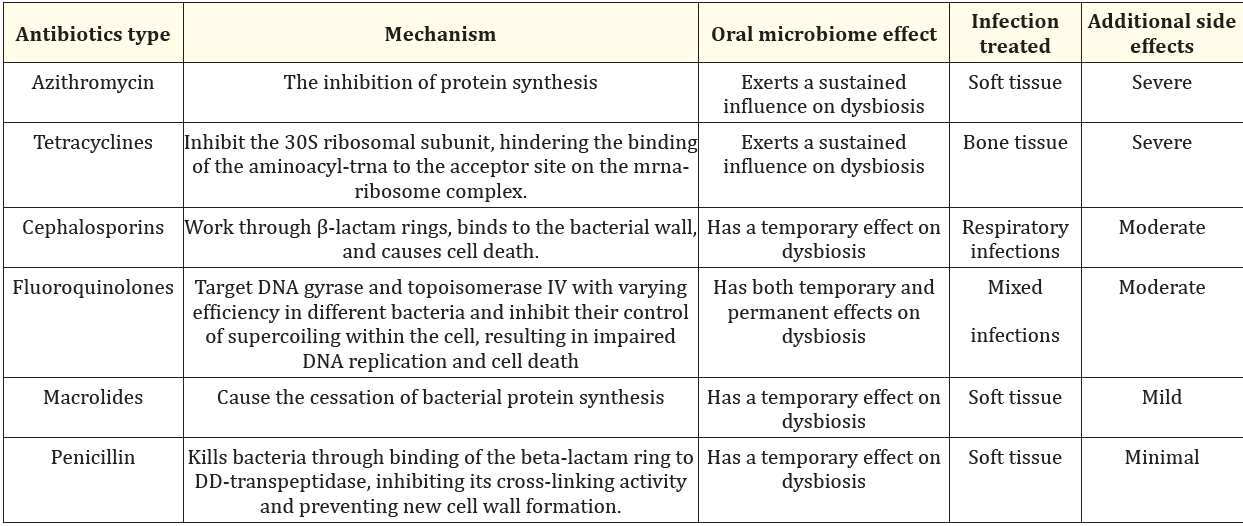
Table 1: The effects of antibiotics on oral microbiome.
Antibiotics have been identified as significant risk factors for HNC patients undergoing IMRT alone or in combination with chemotherapy [63,64]. For instance, a study by Nenclares., et al. [48] revealed that out of 272 HNC patients who received antibiotics one week before and two weeks after treatment, there was a notable earlier progression in their condition. The study also found that these patients had lower overall survival (HR 1.85, P = 0.001) and disease-specific survival (HR 1.95, P = 0.004) rates. Additionally, the study indicated that antibiotic use was significantly linked to a pattern of local and regional relapse rather than distant relapse. It was noted that the negative impact on outcomes was more pronounced in patients who received two or more courses of antibiotics. These findings suggest that regardless of the reason and time of prescription, antibiotic use has a substantially adverse prognostic effect on this group of patients.
Given the considerations mentioned above, the application of antibiotics in patients with HNC undergoing chemotherapy, RT, combined chemo-RT, or immunotherapy carries a potential risk to their survival similar to that observed in other cancer types. The principal hazards associated with the use of antibiotics stem from disturbances in the microbiome and compromised immune function. Although infections, including periodontitis, are frequent in HNC patients, selecting and administering antibiotics prudently is imperative, informed by a comprehensive understanding of their effect on patient prognosis [65-68]. Furthermore, the effective management of periodontitis and other infections, particularly before cancer treatment, is imperative for enhancing overall health outcomes in HNC patients. Given the critical knowledge gap in this area, further research is indispensable to elucidate the precise mechanisms that may inform the development of antibiotics with reduced adverse effects.
In summary, oral health plays a crucial role in the management and outcomes of patients with head and neck cancers (HNCs). Proper oral hygiene before, during, and after cancer therapy prevents complications such as mucositis, infections, and xerostomia, which can harm the patient's quality of life and treatment efficacy. Proactive dental care, encompassing pre-treatment assessments and customized oral hygiene regimens, is imperative in mitigating the adverse effects of radiation and chemotherapy on oral tissues. These measures uphold oral cavity integrity and bolster patient resilience to cancer treatments.
Appropriate antibiotic usage is essential for treating infections affecting the oral cavity, respiratory system, soft tissues, and bones, which commonly stem from compromised immune responses following or during cancer treatment. However, the administration of antibiotics demands careful oversight to reconcile their therapeutic benefits with potential adverse effects, ranging from mild to severe, and their potential impact on patient outcomes, including survival rates. Therefore, the judicious selection of antibiotics and carefully considering administration schedules are imperative to minimize adverse effects and optimize patient outcomes.
In conclusion, integrating comprehensive oral health management and judicious antibiotic administration is paramount for improving HNC patients' treatment success and survival rates. Incorporating preventive oral care and infection control measures into cancer treatment protocols via a multidisciplinary team approach can significantly enhance clinical outcomes and should be considered pivotal.
None.
All authors contributed significantly and equally; and all authors approved the final form of the manuscript.
The authors declare that they have not received any financial support.
All authors contributed significantly and equally; and all authors approved the final form of the manuscript.
Copyright: © 2024 Efsun Somay.,et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.