Diego Fernando López1*, Juliana Ruiz Botero2 and Luis Eduardo Almeida3
1Assistant Professor, Department of orthodontics, Universidad del Valle, Cali, Colombia
2Graduated, Universidad del Valle, Cali, Colombia
3Oral and Maxillofacial Surgeon, Clinical Assistant Professor, Director, Pre-doctoral Program in Oral and Maxillofacial Surgery, Surgical Clinical Sciences, School of Dentistry, Marquette University, Milwaukee, USA.
*Corresponding Author: Diego Fernando López, Assistant Professor, Department of orthodontics, Universidad del Valle, Cali, Colombia.
Received: November 16, 2018; Published: December 05, 2018
Citation: Diego Fernando López., et al. “Early Condylectomy: Biological Considerations”. Acta Scientific Dental Sciences 3.1 (2019): 33-44.
Objective: To describe the early treatment of two patients with unilateral condylar hyperplasia (UCH) diagnosis and therapeutic surgical protocol (early condylectomy) supported by the current biological knowledge about the development and repair of mandibular condyle cartilage (MCC).
Methods: Review of the literature and two case description of the treatment. The two patients were girls, 12 and 13 year old. The follow up period was of 3 years. SPECT diagnostic, radiographic, photographic and tomographic images are presented.
Results: The literature summarized provides biological basis favoring the early treatment of UCH. The two cases were treated with the same surgical protocol obtaining good esthetic and functional stable results.
Discussion: The present literature review and clinical results provide biological basis to justify the practice of early condylectomy. Early intervention appears to be able to produce a successful outcome in terms of esthetic facial symmetry, avoiding dentoalveolar orthodontic compensations or additional orthognathic surgeries.
Keywords: Condylectomy; Facial Asymmetry; Orthodontics; Unilateral Condylar Hyperplasia
Condylectomy is a surgical procedure performed either by preauricular or endo-aural access, exposing the lower components of the temporomandibular joint [1]. The condylar head ostectomy is carried out using a rotatory instrument according to the surgical and therapeutic protocol selected [1,2].
The surgical and therapeutic protocol selection depends of the size of the segment to be eliminated. Therefore, the protocols are classified as: 1) Condylar shaved: about 3 mm removed; 2) high condylectomy: about 5 mm of the condylar head removed from medial to lateral pole; 3) low condylectomy: more than 5 mm removed, and 4) proportional low condylectomy: the length of segment removed is proportional to the extent of the facial asymmetry [1,3]. The protocol selection is also dependent of the pathology, grade of severity and patient´s age.
The etiology of the pathology, time of appearance and kind of abnormal growth of the condylar cartilage are aspects considered for the following classification:
Among the known complications of this kind of pathologies, involving facial asymmetry, are included the abnormal function of respiratory and masticatory system, TMJ pain, skeletal muscle imbalance and psychological effects on adaptation and social relations. These consequences represents the main motives of consult demanding early surgical interventions to reduce the sequelae and reestablish esthetics and function [8,9].
The cartilage of mandibular condyle (CMC), contrary to other cartilages of the cranial base and extremities, is originated from periosteal cells [10-12] and develops adjacent to intramembranous mandibular bone, different to Meckel cartilage [13]. Its morphogenesis occurs in an advanced stage of prenatal development, during the ninth week of intrauterine life, the CMC is considered secondary cartilage, and it is structurally different to other cartilages. It is present in different regions of the craniofacial complex during developmental stages, persisting in the postnatal stage in regions such as the mandibular condyle, the angular process of the mandible and the intermaxillary suture [14,15].
Histologically, the secondary cartilage differs to the primary cartilage in its surface layer, that is, the perichondrium undifferentiated pre-chondroblastic cells that secrete a collagen I rich matrix, different to the collagen II matrix secreted by chondrocytes [16,17]. This is the kind of undifferentiated cells that proliferate and mature to produce CMC growth, instead of the deep layer chondrocytes in the primary cartilage, derived from the embryonal cartilaginous primordium, more genetically determined [16,18,19].
Therefore, the secondary cartilage is able to suffer phenotypic modifications responding to mechanic or functional loads that causes remodeling by an increment in the number of mesenchymal cells or, on the other hand, in absence of function causing a progressive reduction in the hypertrophic layer, reduction of the proteoglycan contents in the matrix, reduction of cartilage thickness and eventually, transformation of the CMC into bone tissue [20,21].
Summarizing the process, condylar neoformation may be dependent on age, articular load, mandibular function and the kind of therapeutic protocol used for treatment [8,21].
The objective of this literature review and cases report is to describe in detail the early treatment of two patients with UCH diagnostic that were treated following a therapeutic surgical protocol (early condylectomy) supported by the current biological knowledge about CMC development and repair.
Female patient 13 year old, consulting for progressive mandibular deviation. Clinically it was observed levognathism, lower dental midline left deviation of 3mm. The patient had bilateral molar dental malocclusion Class I, right canine Class I and left canine Class II (Figure 1).
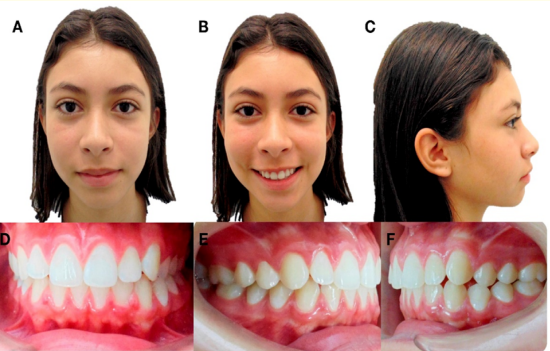
Figure 1: Initial extraoral and intraoral photographs. A) Frontal resting. B) Frontal smiling. C) Profile. D) Frontal intraoral. E) Right Lateral. F) Left Lateral.
In the panoramic radiograph was observed the condylar asymmetry. Bone gammagraphy by Single photon emission computer tomography (SPECT) indicated an increased focal uptake of the radionuclide, of moderate intensity, in the right mandibular condyle, suggestive of osteoblastic hyperactivity. The total counts difference in the uptake index was 12%.
Additionally, in the CT scan it was observed in coronal, sagittal and axial slides and in the 3D bone tissue reconstruction as well, morphologic changes in the right condyle that had higher size and volume than the left condyle (Figures 2,3).
The diagnosis was based on the correlation between clinical intraoral and extraoral findings and radiographic signs. Condylar hyperactivity was confirmed by SPECT results indicating a 12% difference in the uptake of Tc-99m between condyles. This result is indicative of right UCH
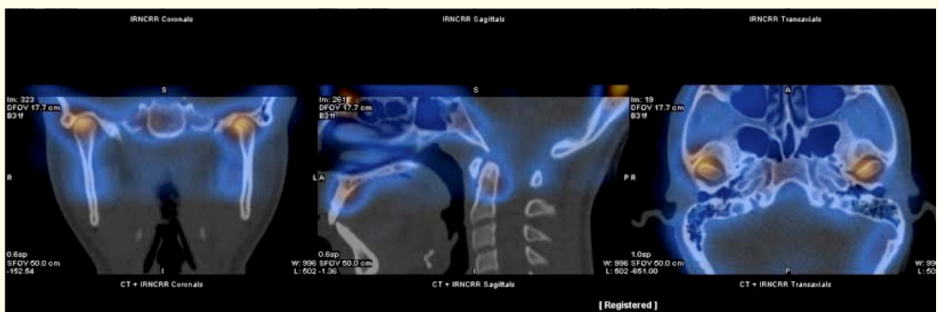
Figure 2: Single photon emission computer tomography (SPECT).
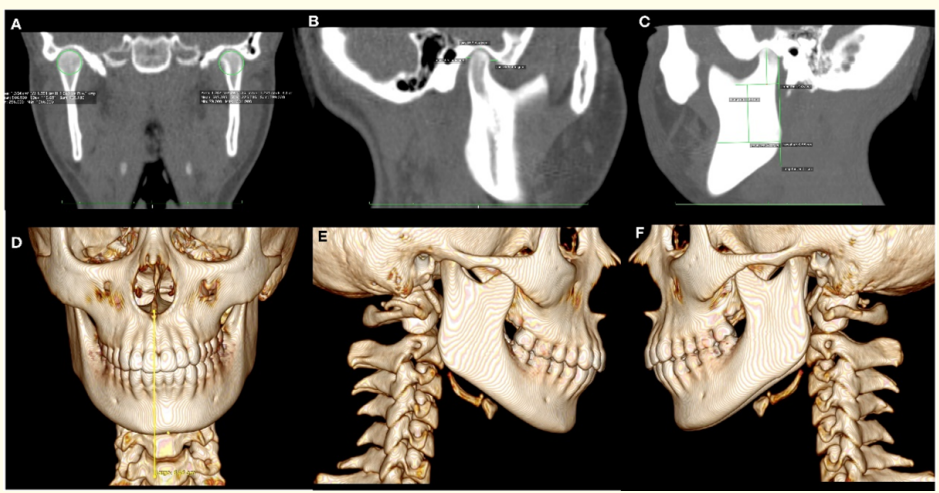
Figure 3: CT. A) Coronal slice showing higher volume in the right condyle. B) Sagittal right slice showing higher length of this condyle. C) Sagittal left slice. 3D Reconstruction of bone tissues. D) Frontal view showing the magnitude of mandibular lateral deviation. E) Right sagital slice presenting only one mandibular body. F) Left sagittal slice presenting two mandibular bodies.
Using upper and lower self-ligation brackets Empower clear AO (American Orthodontics) MBT prescription and TanzoTM CuNiTi .014” archwires, it was initiated the alignment and leveling phase. Then, the patient was treated by early condylectomy. The surgical protocol was by pre-auricular access and the condylar head ostectomy was of 3 mm (conservative condylar shaved).
The specimen of tissue removed was immediately fixed with formaldehyde buffer solution, embedded in paraffin and sectioned to slides of 3 μm, stained with hematoxylin-eosin and Alcian Blue (Figure 4).
The histopathology report showed chondrocyte islands, width of the proliferative layer plus cartilage layer, 0.4 mm and total thickness of 0.6 mm in the soft condyle. This size is indicative of a significant increase compared to the normal reference values (respectively 0.29 ± 0.064 and 0.46 ± 0.104), a result considered as positive for UCH of the right condyle.
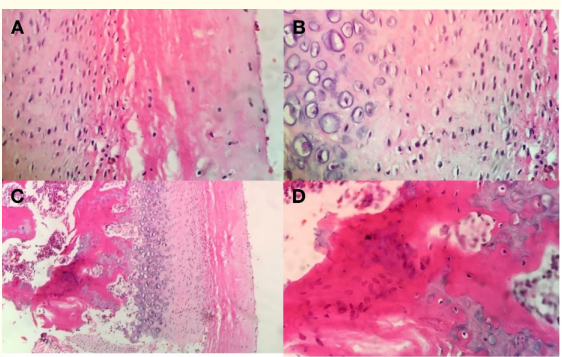
Figure 4: A) Fibrous articular layer, B) Proliferative layer, C) Hyperplasic cartilage layer, D) Islands of cartilage.
After the surgical procedure, the orthodontic treatment was continued by using intermaxillary elastics of ¼ and 2.5 ounces in the first appointment and 4.5 ounces afterwards. The elastics used are asymmetric with a class III right vector and class II left vector, in order to improve the seating of the right condyle treated, into a position up and backwards within the articular fossa to correct the open bite that is frequently present following a condylectomy (Figure 5).
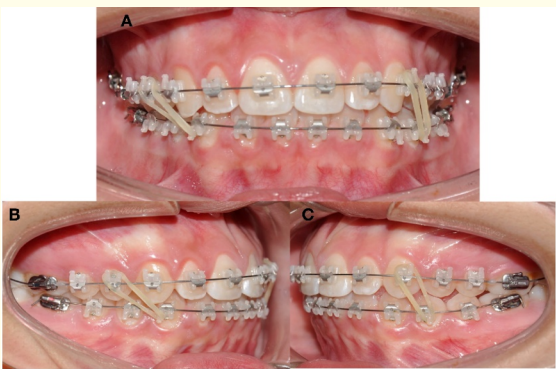
Figure 5: Elastic mechanics. A) Intraoral frontal view. Brackets Empower clear AO (American Orthodontics) MBT prescription. B) Lateral right intermaxillary elastic ¼ 2.5 ounces Class III vector. C) Lateral left intermaxillary elastic ¼ 2.5 ounces Class II vector
After the use of elastics was suspended it was followed with thermoactivated archwires TanzoTM CuNiTi 0.018” and TanzoTM CuNiTi 019” × .025”. The orthodontic treatment was finished with stainless steel upper and lower archwires AO .019” x .025” (Figure 6).
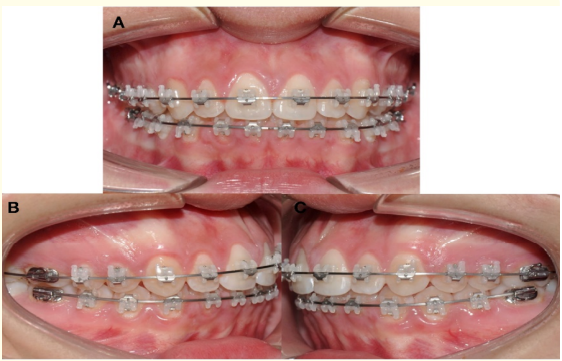
Figure 6: Stainless steel upper and lower archwires AO .019x .025”. A) Frontal view. B) Right view. C) Left view.
The orthodontic treatment was finalized in 12 months. The mid sagittal plane deviation was corrected and molar and canine class I, anterior teeth coupling, and adequate overjet and overbite were obtained. After the appliance removal, the tomographic control showed correct healing and CMN neo-formation in the right condyle. The open mouth final tomography did not show midline deviation (Figures 7,8).
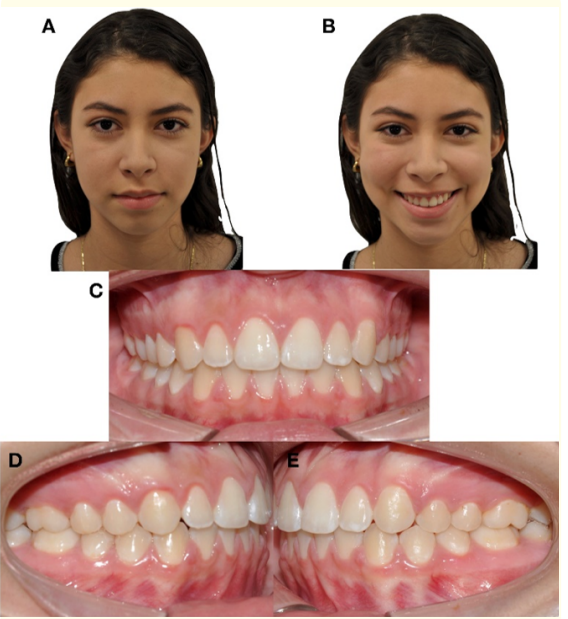
Figure 7: Extraoral and intraoral final photographs. A) Frontal resting. B) Frontal smiling. C) Frontal intraoral.
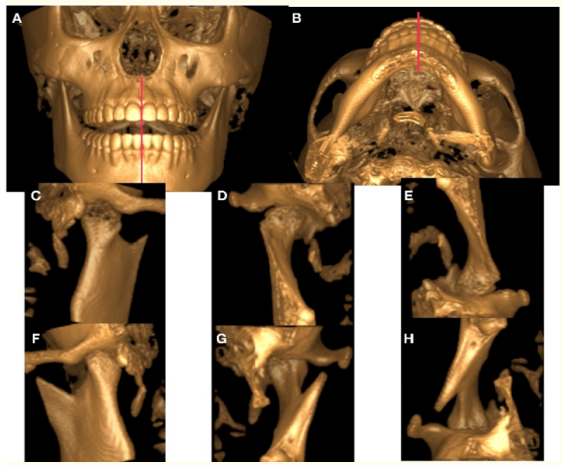
Figure 8: Control CT. 3D reconstruction of bone tissues taken at the end of treatment with open mouth. A) Frontal View. B) Axial View. 3D reconstruction of bone tissue from different angles to show the condyle after 12 months of condylectomy. C) Posterior View. D) Frontal View. E) Axial View. Contralateral condyle F) Posterior View. G) Frontal view. H) Axial View.
Female patient 12 year old, consulting for progressive mandibular deviation, with no TMJ symptoms. Clinically, it was observed dextrognathism with deviation of the inferior dental midline 3 mm to the right, molar and right canine class I and left molar and left canine class III (Figure 9).
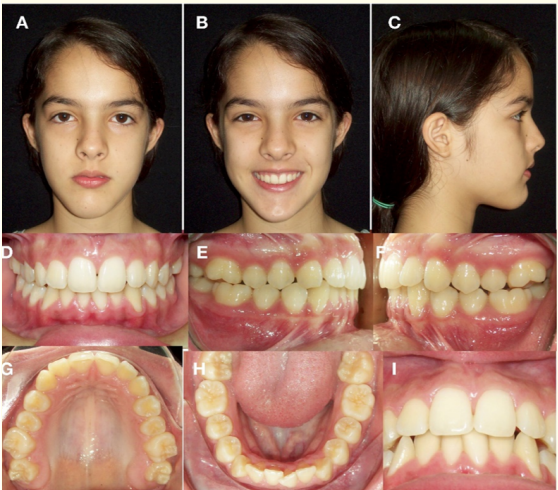
Figure 9: Initial extraoral and intraoral photographs. A) Frontal resting. B) Frontal smiling. C) Profile. D) Frontal intraoral. E) Right Lateral. F) Left Lateral.
In the panoramic radiography it was observed the left condyle slightly increased in size. The SPECT gammagraphy shows an increment in the uptake of the radionuclide, focal and of moderate intensity (difference of 12% positive for the left mandibular condyle), indicative of increased osteoblastic activity for this condyle (Figure 10).
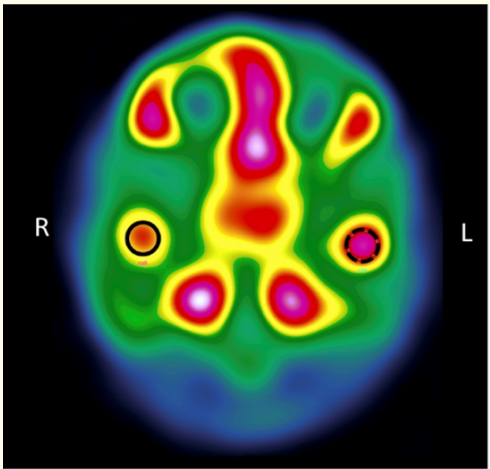
Figure 10: Single photon emission computer tomography (SPECT).
The diagnosis was based on the correlation between clinical intraoral and extraoral findings and the radiographic signs. Condylar hyperactivity was confirmed by SPECT results indicating a 12% difference in the uptake of Tc-99m between condyles. This result is indicative of left UCH.
Upper and lower Mini twin - Ortho Organizer™ brackets with archwires Super Elastic Nitanium® .014” were cemented and then the maxillofacial surgery was performed. Then, the patient was treated by early condylectomy. The surgical protocol was by preauricular access and the condylar head ostectomy was of 3 mm (conservative condylar shaved).
The histopathology treatment and interpretation were the same described in Case 1 (Figure 11).
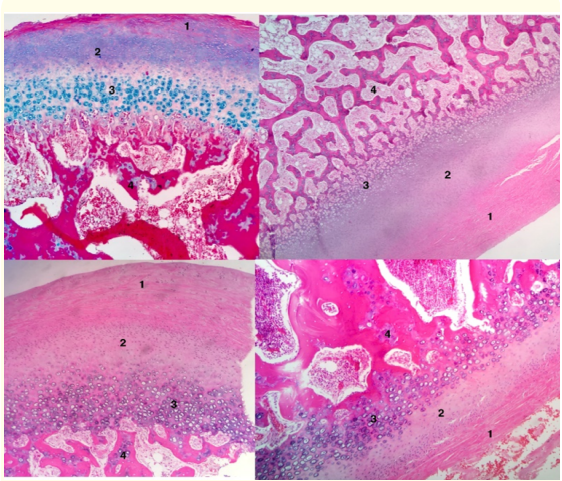
Figure 11: Fibrous articular layer (1). Proliferative layer (2). Hyperplasic cartilage layer (3). Islands of cartilage (4).
Following the surgical procedure, the orthodontic treatment was continued using intermaxillary elastics of ¼, 2.5 ounces in the first appointment and 4.5 ounces afterwards. The elastics were asymmetric with a class III left vector and class II right vector, in order to improve the seating of the left condyle treated, into a position up and backwards within the articular fossa to correct the open bite that is frequently present following a condylectomy (Figure 12).
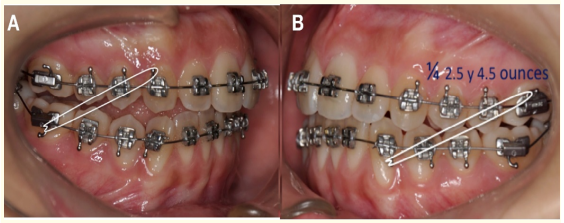
Figure 12: Elastic mechanics. A) Lateral right intermaxillary elastic ¼ 2.5 ounces Class II vector.
The orthodontic treatment was continued with Super Elastic Nitanium® .018” archwires and as it was observed the coincidence of the dental midlines it was changed to Super Elastic Nitanium®.019” × .025”. The arch sequence was continued with MASEL®.019” × .025” stainless steel upper and lower archwires. The final upper and lower arch was .019” × .025” stainless steel multifilament threaded.
After 12 months of orthodontic treatment it was corrected the mid sagittal plane deviation and it was obtained a bilateral molar and canine class I, anterior teeth coupling with adequate overjet and overbite. After removing the appliances, the tomographic control showed adequate healing and CMC neo-formation in the left condyle (Figures 13,14). The open-mouth tomography showed adequate range of mouth opening without deviation in the maximum range of opening.
The occlusion was stabilized in both cases using retainers. Three years after the end of orthodontic treatment the control shows stable occlusion and symmetry (Figure 15).
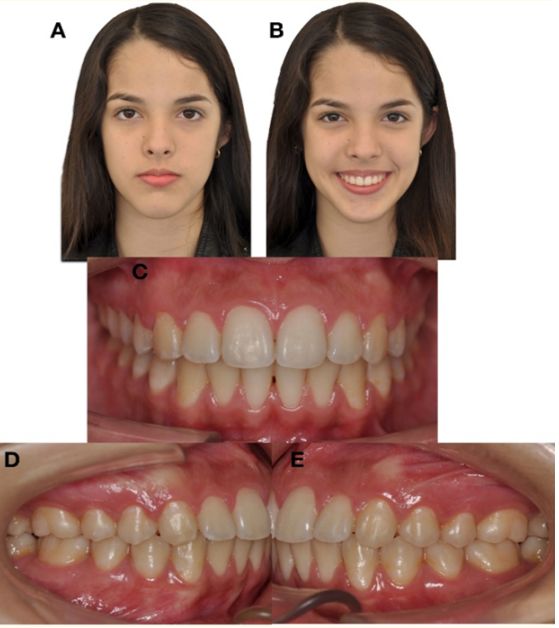
Figure 13: Final extraoral and intraoral photographs. A) Frontal resting. B) Frontal smiling. C) Frontal intraoral. D) Right Lateral. E) L

Figure 14: Control CT. 3D reconstruction of bone tissues at the end of treatment. with open mouth. A) Frontal view. B) Contralateral condyle - sagittal view. C) Condyle after 12 months of condylectomy sagittal view.
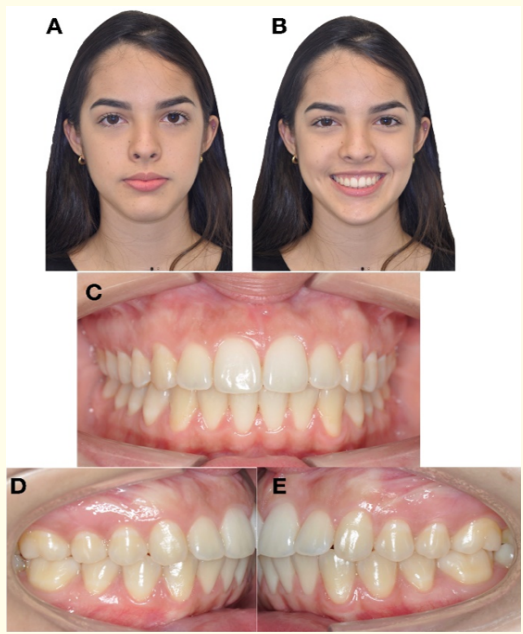
Figure 15: Extraoral and intraoral photographs. Control of retention 3 years after treatment A) Frontal resting. B) Frontal smiling. C) Frontal intraoral. D) Right Lateral. E) left Lateral.
UCH diagnosis must be made correlating the facial and occlusal clinical parameters with tomographic/radiographic findings [22,23]. Once established the clinical diagnostic and suspecting that the overgrowth process is active, the nuclear medicine test is the recommended confirmatory procedure. SPECT is the test that has more ability to detect hyperactivity of the condylar tissue compared to the contralateral condyle [24]. A difference in uptake between condyles higher than 10% is the accepted cut off indicative of UCH [25].
In general, when the active state of UCH is detected, it is recommended to perform a condylectomy in order to avoid the advance of the disorder and the development of facial, occlusal and functional sequelae. The condylectomy protocol to follow is part of the therapeutic decision. The specimen removed is sent to pathology to evaluate cellular characteristics and confirm the diagnosis. In hyperplasic condyles there is an increased width in the soft condyle layers due to the chondrogenic and osteogenic hyperactivity.
During and after the surgical procedure some complications are expected, including: bleeding due to injury of the superficial temporal artery or the internal maxillary artery, edema, facial nerve injury (cut or compression) and displacement of the segment removed to the medial region [26]. Post-surgical complications are related to infection, cheloid healing, difficult lift of the eyelid and the superciliary zone if the frontal nerve is injured and reduced sensibility in the same zone. Additionally, cases of Frey syndrome (hyperhidrosis when eating, due to injury to the auricular temporal nerve) are reported [27,28].
Occlusal complications are consistently reported in connection to condylectomy. They include open mouth due to anterior-inferior rotation of the condyle induced by hemarthrosis, release of the stabilizing retrodiscal tissue and stretching of the upper external pterygoid bundle [29].
The decision to practice early condylectomy is controversial, basically due to the unknown possibility of amputation of the condylar growth center, reducing the post-surgical growth of the operated condyle while the other condyle grows more, producing a secondary asymmetry. However, Brusati., et al. [30], and Di Blassio., et al. [31] provide evidence of postsurgical condylar growth that may be even higher than the healthy condyle growth.
The mandibular condyle cartilage is lately developed, during the 8th to 12th week of intrauterine life because it is a secondary cartilage. During the 8th week a mesenchymal condensation forms the condylar blastema that is the anatomic structure for insertion of the lateral pterygoid muscle [19].
Later on, during the 9th week endochondral ossification takes place, involving the condylar blastema by cartilaginous tissue (soft condyle), due to migration and differentiation of mesenchymal cells to chondrocytes that secrete the extracellular matrix of cartilage rich in collagen type II [18].
Thus, the condyle as secondary cartilage grows and ossificates quickly stimulated by the activity of the lateral pterygoid muscle [32]. During the 12th week the head of the condyle develops a conical – elongated shape that by epigenetic ways stimulates the development of the glenoid cavity and other TMJ components [19].
The CMC secondary cartilage, contrary to the primary cartilage, is not strictly genetically controlled. Therefore, the total mandibular length is not genetically determined, and epigenetic stimuli may determine its future development [19].
The CMC is not determined by primary influences and is not covered by a cartilaginous matrix that isolates or protects it from local or external factors [18,19]. Therefore, its phenotype is able to change by mechanic or functional factors. This explains the effect on the condyle of local factors such as occlusion, masticatory function, orthopedic and orthodontic appliances, orthognatic surgery as well as parafunctional activities and trauma.
External local factors may modify the rate and amount of growth of the CMC through the action of signals such as the Indian Hedgehog (IHH) reported by Tang., et al. [21] as mechanotransductor stimulating the proliferation of chondrocytes.
CMC cells are highly dependent of the mandibular movement and as the TMJ is an articulation constantly active its growth is granted, but if it is reduced or eliminated, may be able to change from cartilage to osteogenic phenotype.
Histologically, the condyle is formed by a Surface layer of fibrous connective tissue, a proliferative layer of mesenchymal cells, a layer of hypertrophic cartilage (soft condyle), and finally by an ossification layer. Eslami., et al. [33], reported an average width of the soft condyle of 0.46 mm in healthy condyles.
Gray., et al. [34] and López., et al. [35] described the histologic characteristics of hyperplasic condyles and the width of soft condyle layers, finding maximum values of 1.12 mm and 0.9 mm respectively.
Fariña., et al. [36] reported the depth of cartilage islands in trabecular bone, with a maximum extension of 1.7 mm. These islands are a consistent representative characteristic found in growing condyles.
The proliferative activity due to increased chondrogenesis and increased condylar osteogenesis, and consequently condylar osteogenesis is developed up to a maximum depth of 3 mm in hyperplasic condyles. Therefore, the surgical procedure in teenage patients, with a potential growth and development, when the pathology is detected in its initial stage and in cases of mild asymmetry and esthetic sequelae, should be limited to the elimination of 3 mm of condylar surface (soft condyle) as its was performed in the 2 cases described [37].
The blood vessels neoformation after the surgical procedure is a natural process during injury healing and provides oxygen, nutrients and undifferentiated mesenchymal cells to prevascular tissues able to differentiate to chondrocyte and osteoblast precursors that will develop bone in growing condyles and during the repair stages [38].
The vascular endothelial growth factor (VEGF) is known as a potent mitogen factor in endothelial cells and angiogenesis promotor during skeletal development, post-natal growth and tissue homeostasis [39, 40]. Additionally, it participates in the healing process, after condylar surgery [41].
The functional demands increase the population of mesenchymal undifferentiated cells of the proliferative layer and the increment in the population of chondrocytes and its development in the hypertrophic zone induces the recruiting of osteoblasts and chondroblasts in the eroded (operated) zone. This process is stimulated by the Fibroblast growth factor (FGF) that promotes mitosis and differentiation to chondroblasts [42]. In turn, chondroblasts are stimulated by the transcription factor SOX 9 that controls chondrocyte differentiation and regulates the synthesis of collagen II, X and XI to form cartilage [43,44].
Following the production of cartilage matrix, the chondrocytes initiate the hypertrophic process mediated by the protein RUNX2 that activates the synthesis and secretion of collagen X. This kind of collagen is ideal to form hypertrophic matrix in the cartilage that if it is eroded will be replaced by bone tissue [45,46].
Neovascularization of hypertrophic cartilage is also mediated by VEGF that in turn activates the endochondral ossification process permitting condylar growth [47].
During the processes of endochondral ossification and bone tissue healing, it is also occurring the process of trans-differentiation, defined as a process of differentiation changing the phenotype and function in mature cells. In the classic model of endochondral ossification and during the repair of fractures and injuries, it is observed that hypertrophic chondrocytes are able to change their morphology by fragmentation of the DNA pattern. This is one of the mechanics for differentiation to osteoblasts and to osteocytes during post-natal bone formation, mediated by the protein Osterix that also regulates the formation of subchondral bone during CMC growth [48].
The condylar cartilage is essential in the hierarchy of TMJ development because the disk and fossa is not formed if CMC is absent. It has been suggested that space correlation between anatomic structures is able to determinate the craniofacial conformation [49]. It is also hypothesized that the kind of articulation existing between temporal, occipital and parietal bones, reflex the forces generated during mastication that are distributed along the cranium. This indicates that the mandible and temporal bones reciprocally modify their position and movement, acting as a unit [49-51].
Changes in the position of the glenoid fossa during growth may be able to have an influence in the development of malocclusions and facial asymmetries. On the other hand, the position of the glenoid fossa may be determined by the anatomy and function of the mandibular condyle, as well as by the occlusion and dental position [51]. Compensatory and adaptive processes according to function (Mastication, swallowing, occlusion, orthopedic and muscular forces, are able to modify the articular anatomy).
The present literature review and clinical results provide biological basis to justify the practice of early condylectomy.
The embrionic origin of the mandibular condyle, the adequate selection of the surgical approach, the biology of healing processes mediated by angiogenesis, endochondral ossification and the ability of articular tissues to adapt and being remodeled, justify the surgical method of condylar shaved on the condition that this therapeutic decision is based on a rigorous diagnostic. Early intervention appears to be able to produce a successful outcome in terms of esthetic facial symmetry, avoiding dentoalveolar orthodontic compensations or additional orthognathic surgeries.
Longitudinal studies with extensive follow up are necessary to evaluate all the variables involved in craniofacial growth and provide further evidence supporting these conclusions.
The authors report no conflicts of interest
This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Copyright: © 2019 Diego Fernando López., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.