Shaimaa Rabea Mohamed1*, Jealan Mohamed El Shafei2, Mohamed Hussein Zaazou3 and Alaa Abd El Salam El Baz4
1Assistant Researcher, Restorative and Dental Material Department, National Research Center, Dokki, Giza, Egypt
2Professor of Endodontics, Endodontic department, Faculty of Oral and Dental Medicine, Cairo University, El Manial, Cairo, Egypt
3Professor of Restorative Dentistry, Restorative and Dental Material Department, National Research Center, Dokki, Giza, Egypt
4Associate Professor of Endodontics, Endodontic department, Faculty of Oral and Dental Medicine, Cairo University, El Manial, Cairo, Egypt
*Corresponding Author: Shaimaa Rabea Mohamed, Assistant Researcher, Restorative and Dental Material Department, National Research Center, Dokki, Giza, Egypt.
Received: November 19, 2018; Published: December 04, 2018
Citation: Shaimaa Rabea Mohamed., et al. “Assessment of the Influence of Radiotherapy on the Effectiveness of Root Canal Irrigation for Smear Layer Removal (A Comparative In-Vitro Study)”. Acta Scientific Dental Sciences 3.1 (2019): 12-20.
The purpose of the study was to evaluate the influence of gamma-irradiation on the effectiveness of root canal irrigation for smear layer removal. Methods: A total of fifty recently extracted single rooted human permanent teeth were used and divided into 2 main groups according to exposure to irradiation: Group (I): Non radiotherapy: (n = 15) no irradiation. Group (II): Radiotherapy: (n = 35) those subjected to a total dose of 60 gray of radiation in fractions of 2 gray/day five days a week for six weeks. The samples of each group were then subdivided into 3 subgroups according to the irrigating solution used as final rinse into: Subgroup A: Saline (Control group) (n=10, 5 for each group), Subgroup B: 17% EDTA (n = 5 for Group I, n = 15 for Group II), Subgroup C: MTAD (n = 5 for Group I, n = 15 for Group II). The teeth were decoronated and the root canals were instrumented and irrigated. The teeth were then split longitudinally and examined under SEM (X2000) for presence or absence of smear layer. The data were collected and statistically analyzed. Results: ANOVA test revealed that Group II had a statistically significantly higher mean rank of smear layer scores than Group I. Conclusion: within the limitations of this in-vitro study, it could be concluded that, the irradiation dose (60 gray delivered in fractions of 2 Gy/day) had significantly reduced the debridement efficiency of the irrigating solutions at the coronal and middle thirds. Both EDTA and MTAD treated samples in Group I were equally effective in smear layer removal at the coronal and middle thirds. On the contrary, both were inefficient in removing smear layer at the apical third.
Keywords: EDTA; Irrigation; MTAD; Radiotherapy; Smear Layer; SEM
Gy: Gray (unit of the irradiation dose); ‘EDTA’: Ethylenediamintetra-Acetic Acid; BioPure MTAD: A Mixture of Doxycycline, Citric Acid, and A Detergent [Tween 80]; NaOCl: Sodium Hypochlorite; SEM: Scanning Electron Microscope; Tween 80: Polyoxyethylene Sorbitan Monooleate.
A successful endodontic treatment can be accomplished by proper debridement, disinfection and afterwards creating an impermeable seal for the root canal system against bacterial ingress. Complete disinfection of the pulp space cannot be achieved with most intellectual instrumentation techniques. Therefore, irrigation is an essential part of debridement and its role in obtaining this objective cannot be undervalued. Ethylenediamintetra-acetic acid ‘EDTA’ is a chelating agent proved to be capable of removing inorganic material and the smear layer [1], cleaning and helping in disinfection of the canal. BioPure MTAD (a mixture of doxycycline, citric acid, and a detergent [Tween 80]) showed the capability to remove the smear layer safely and completely with minimal erosion of dentinal tubules [2].
Nowadays, patients suffering from malignancies are in a progressive rise and these patients may require endodontic treatment [3]. Secondary effects on oral and perioral tissues can result from the therapeutic radiation applied to treat malignancies in the head and neck region. For instance, high risk to radiation caries is a frequent consequence to salivary gland malfunction of the irradiated patient [4]. Regarding carious lesions with pulp involvement in non-irradiated normal patient, a couple of therapeutic options exist: endodontic therapy or exodontia. Osteoradionecrosis is a common after-effect of exodontia in patients formerly subjected to irradiation. As a result, whenever possible any attempt for exodontia following radiotherapy has to be precluded. Alternatively, root canal treatment should be recommended to avoid any trauma and incidence of osteoradionecrosis [5-7]. In addition, ionizing radiation may have an effect on dentin microhardness and bond strength of dental adhesives to dentin [6-8]. Therefore, the aim of this study is to evaluate the influence of gamma-irradiation of teeth on the effectiveness of root canal irrigation to remove the smear layer using scanning electron microscope.
Fifty recently extracted single rooted human permanent teeth with straight root canals and completely formed apices were collected. Teeth were divided into 2 main groups according to exposure to irradiation: Group I: Non-radiotherapy group; the teeth of this group (n = 15) were not subjected to irradiation before irrigation. Group II: Radiotherapy group; the teeth of this group (n = 35) were subjected to a total dose of 60 gray (Gy) of irradiation before irrigation in fractions of two Gy/day (conventional fractionation schedule) five days a week for six weeks. The samples of each group were then subdivided into three subgroups according to the type of irrigating solution used. Subgroup A: Root canals “control” (n = 10) were irrigated with saline. Subgroup B: Root canals (n=20) were irrigated with 5.25% NaOCl as intracanal irrigating solution and finally 17% EDTA was used as a final rinse for removal of the smear layer. Subgroup C: Root canals (n = 20) were irrigated with 5.25% NaOCI as intracanal irrigating solution and finally MTAD was used as a final rinse for removal of the smear layer.
Teeth receiving radiation were placed in a 25-square-centimeter plastic container containing saline solution during irradiation. The saline solution was replaced daily to a level up to 0.5 cm above the teeth in the plastic container. Radiation was administered with Co-60 photons (Theratron 780E, Theratronics Int., Carrollton, Texas) by using a single anterior field. The source liquid surface distance was 80 cm. A total dose of 60 gray of irradiation in fractions of 2 Gy/day five days a week for six weeks was delivered. After irradiation, all teeth were stored in saline.
The lengths of the teeth were standardized in all specimens. The pulp tissue was extirpated with a barbed broach (Dentsply Maillefer) from the root canal. A size 10 K- type file (MANI INC. Japan) was used to verify patency of the canal and apical foramen. A small amount of pink wax (Kerr, USA) was placed over the apex of each root to block the apical foramen during irrigation. This was done after placing a calibrated gutta-percha cone (MTA-Kore) at the working length. The cone was removed after the wax had set. All root canals were instrumented using step-back technique. Root canals were irrigated with two ml of 5.25% NaOC1 between each instrument except subgroup (A) where saline was used as an irrigating solution between each instrument. The irrigant was delivered with an endodontic syringe with a 30 gauge blunt needle with closed rounded end and side port dispersal (Max-i-Probe: Dentsply Rinn, USA). After complete instrumentation, the canals were treated with one of the experimental irrigating solutions as a final rinse: subgroup (A): Saline, subgroup (B): 17% EDTA and subgroup (C): MTAD. The needle was passively placed in the canal space one to two mm short of working length. The needle outlet in all irrigation subgroups was placed towards the buccal surface of the root all times. One ml of the irrigating solution was slowly delivered into the canal using up and down motion. A # 15 hand file wrapped with gauze was placed to working length and mechanically agitated the solution. The solution allowed to stay in the canal for five minutes, then removed with suction, the canals were then rinsed with the remaining four ml of the solution. The canals were then dried with compressed air and absorbent paper points (MTA-Korea).
A cotton pellet was held at the orifice of each canal. All roots were grooved longitudinally on the external surface with a diamond disc, avoiding penetration of the root canals. The roots were then carefully split with a mallet and chisel (ACE surgical,MA, USA). One half of each root was selected; the root half selected for evaluation was chosen randomly according to the best visibility of the canal. A scanning electron microscope (SEM) was used for evaluation of root canal cleanliness after canal irrigation. A magnification of (X-2000) was used for SEM evaluation. Each selected half was attached to a coded stub. The specimens were placed in a vacuum chamber and then gold sputtered and observed with a SEM (JEOL, Japan).
A general survey of the canal wall from the apex to the crown was done. Each third was then carefully examined, divided by two vertical lines and a photomicrograph of the central part was obtained at a standard magnification of (X-2000) of each third. A photomicrograph (X-2000) of those areas representative of the dominant condition of each third per specimen was taken. A total of three photomicrographs per specimen were taken. Each photomicrograph was divided into 16 equal parts (rectangles). Each rectangle was evaluated separately according to the scoring system [2] which depends on presence/absence of smear layer and visualization of the entrance to dentinal tubules. Smear scores: Score 0: no smear layer on the dentine wall, all tubules opened. Score 1: light smear layer, with more than 50% tubules opened. Score 2: moderate smear layer, with less than 50% tubules opened. Score 3: heavy smear layer, with outlines of tubules obliterated. Score 4: very heavy smear layer, with outlines of tubules disappeared. Then the mean ranks for the smear layer scores at the coronal, middle and apical levels in each group were evaluated.
Data were presented as mean rank for smear layer scores. A non-parametric one-way ANOVA (Kruskal–Wallis) test followed by paired group comparisons using Mann–Whitney U tests at a 5% significance level were used to analyze the smear layer scores statistically for the effect of radiotherapy, irrigant and the difference between tested root third. Statistical analysis was performed with IBM® SPSS® Statistics Version 21 for Windows.
At the coronal and middle thirds of subgroup B; ‘5.25% NaOCl + 17% EDTA treated samples’ and subgroup C; ‘5.25% NaOCl + MTAD treated samples’ the results revealed that: Group II ‘radiotherapy group’ (Figure 2,4,8,9) had a statistically significantly higher mean rank of smear layer scores than Group I ‘non-radiotherapy group’ (Figure1,3,7,10). While at the apical third, there was no statistically significant difference in the mean rank of smear layer scores between both groups (Figure 5,11,6,12) (Table 2 and 3).
Subgroup A; ‘Saline treated samples’ showed the statistically significantly highest mean rank of smear layer scores as shown in table 1. This was followed by subgroup B and subgroup C with no statistically significant difference in the mean rank of smear layer scores between them in each group (Table 2 and 3).
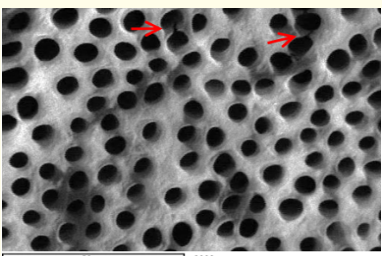
Figure 1: A SEM photomicrograph for the coronal third of a non-irradiated ‘5.25% NaOCl + EDTA’ irrigated root canal surface “Group I, subgroup B” showing a clean, debris free surface with many visible dentinal tubule’s open
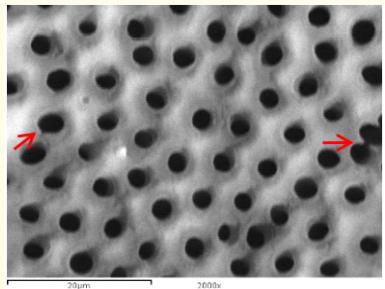
Figure 2: A SEM photomicrograph for the coronal third of an irradiated ‘5.25% NaOCl + EDTA’ irrigated root canal surface “Group II, subgroup B” showing a clean, debris free surface with many visible dentinal tubule’s openings and areas of erosion (X 2000).
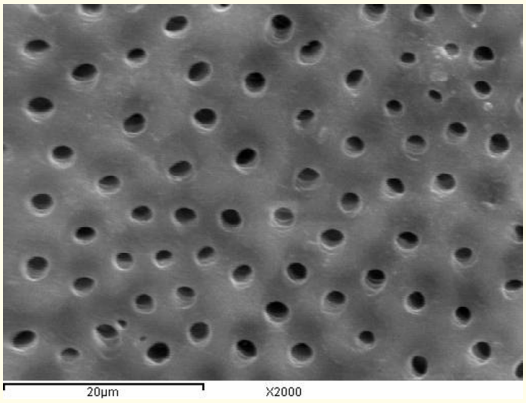
Figure 3: A SEM photomicrograph for the middle third of a non-irradiated ‘5.25% NaOCl + EDTA’ irrigated root canal surface “Group I, subgroup B” showing a clean, debris free surface with visible dentinal tubules openings and areas of erosion (X 2000).

Figure 4: A SEM photomicrograph for the middle third of an irradiated ‘5.25% NaOCl + EDTA’ irrigated root canal surface “Group II, subgroup B” showing a clean, debris free surface with many visible dentinal tubule’s openings and areas of erosion (X 2000).
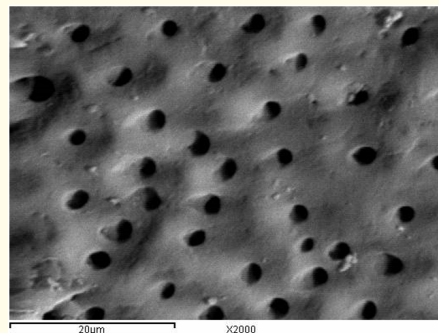
Figure 5: A SEM photomicrograph for the apical third of a non-irradiated ‘5.25% NaOCl + EDTA’ irrigated root canal surface “Group I, subgroup B” showing an almost clean surface, with visible dentinal tubules openings (X 2000).

Figure 6: A SEM photomicrograph for the apical third of an irradiated ‘5.25% NaOCl + EDTA’ irrigated root canal surface “Group II, subgroup B” showing an almost clean surface, with visible dentinal tubules openings (X 2000).
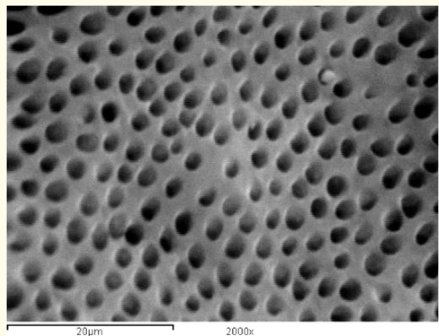
Figure 7: A SEM photomicrograph for the coronal third of a non-irradiated ‘5.25% NaOCl + MTAD’ irrigated root canal surface “Group I, subgroup C” showing a clean, debris free surface with many visible dentinal tubule’s openings (X 2000).

Figure 8: A SEM photomicrograph for the coronal third of an irradiated ‘5.25% NaOCl + MTAD’ irrigated root canal surface “Group II, subgroup C” showing a clean, debris free surface with many visible dentinal tubule’s openings (X 2000).
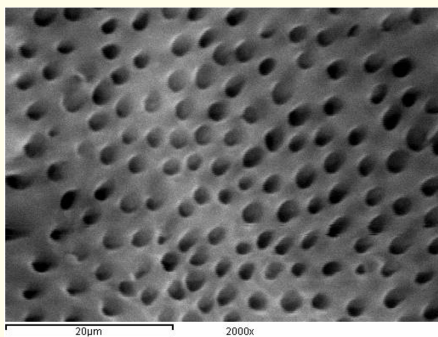
Figure 9: A SEM photomicrograph for the middle third of a non-irradiated ‘5.25% NaOCl + MTAD’ irrigated root canal surface “Group I, subgroup C” showing a clean, debris free surface with visible dentinal tubules openings (X 2000).
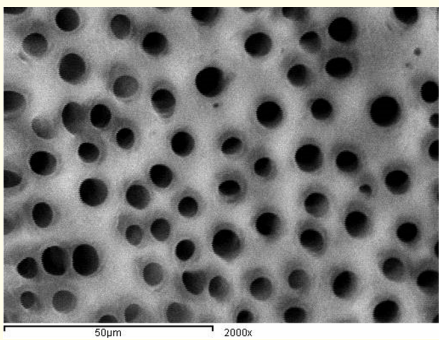
Figure 10: A SEM photomicrograph for the middle third of an irradiated ‘5.25% NaOCl + MTAD’ irrigated root canal surface “Group II, subgroup C” showing a clean, debris free surface with visible dentinal tubules openings (X 2000).
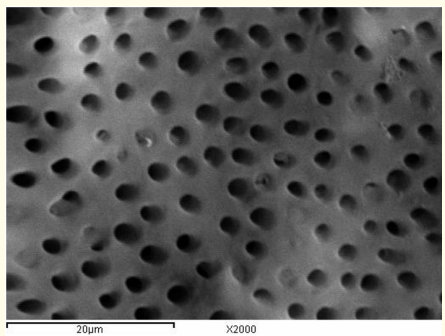
Figure 11: A SEM photomicrograph for the apical third of a non-irradiated ‘5.25% NaOCl + MTAD’ irrigated root canal surface “Group I, subgroup C” showing an almost clean, debris free surface with visible dentinal tubules openings (X 2000).
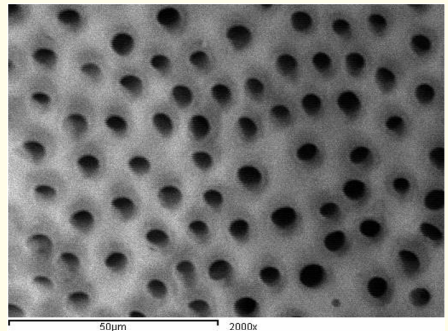
Figure 12: A SEM photomicrograph for the apical third of an irradiated ‘5.25% NaOCl + MTAD’ irrigated root canal surface “Group II, subgroup C” showing a clean, debris free surface with visible dentinal tubules openings (X 2000).
The apical third in subgroup B and subgroup C showed the statistically significantly highest mean rank of smear layer scores. This was followed by the middle and coronal thirds with no statistically significant difference in the mean rank of smear layer scores between them in each group (Table 2 and 3).
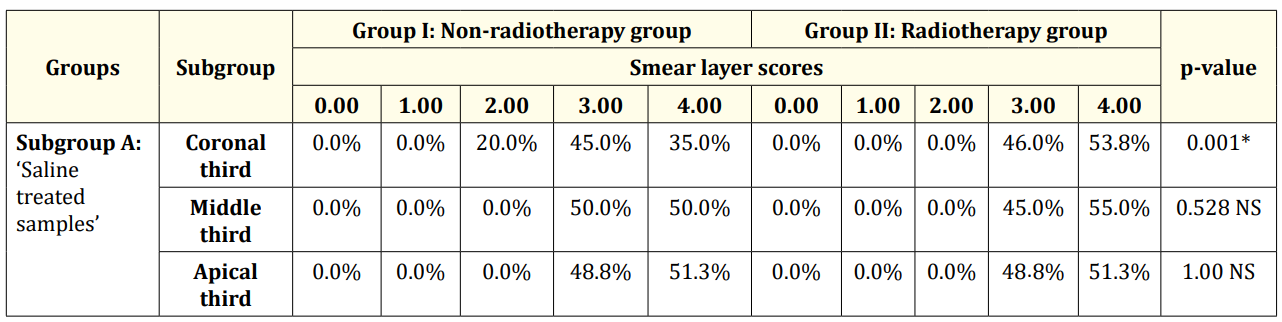
Table 1: Descriptive statistics and test of significance difference for the percentages of smear layer scores between Group I and II (subgroup A). *Significant at P≤ 0.05, NS: non-significant at P > 0.05.
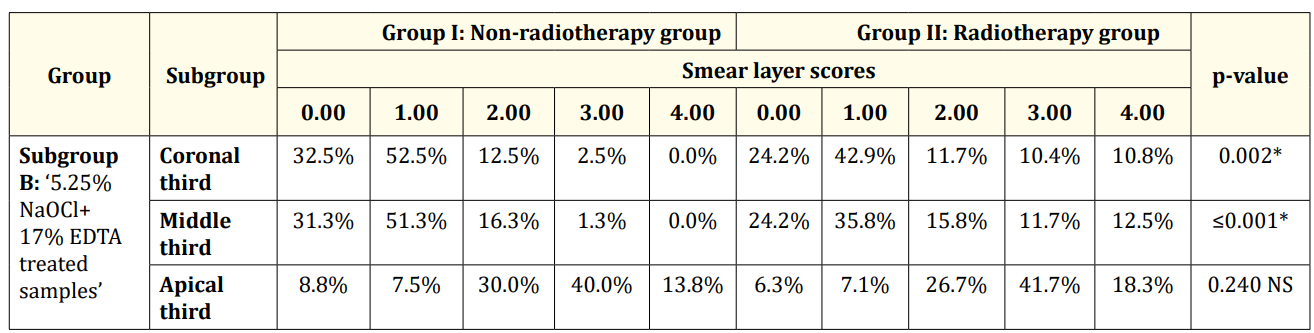
Table 2: Descriptive statistics and test of significance difference for the percentage of smear layer scores between Group Iand II (subgroup B).
Smear scores:
Score 0: no smear layer on the dentine wall, all tubules opened.
Score 1: light smear layer, with more than 50% tubules opened.
Score 2: moderate smear layer, with less than 50% tubules opened.
Score 3: heavy smear layer, with outlines of tubules obliterated.
Score 4: very heavy smear layer, with outlines of tubules disappeared.
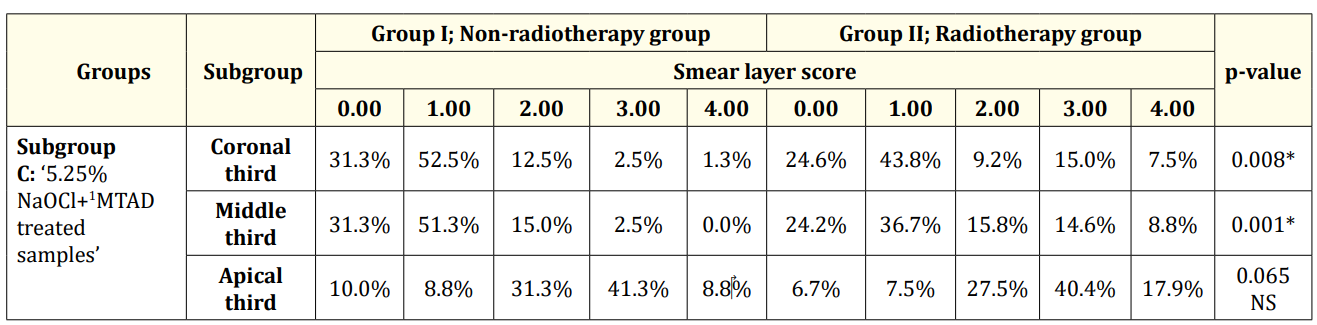
Table 3: Descriptive statistics and test of significance difference for the percentage of smear layer scores between Group Iand II (subgroup C).
The current study was carried out in an attempt to assess the effect of radiotherapy on the effectiveness of root canal irrigation for smear layer removal.
Radiotherapy “Radiation therapy” is the treatment of cancer with ionizing radiation. The goal of radiation therapy is to maximize the dose to abnormal cells while minimizing exposure to normal cells [8].
The irradiation dosage in the head and neck region usually ranges between 40 and 70 Gy [9]. In this study, the specimens were irradiated with a total dose of 60 Gy of irradiation in fractions of 2 Gy/day five days a week for six weeks. The dose was defined on the radiotherapy unit panel that self-measures the radiation level emitted, which corresponds to a common clinical procedure for adults receiving radiotherapy [6,7].
In the current study, a daily dose rate of (2 Gy/min) was delivered to the specimens. This is to mimic the conventional fractionation schedule. Exposure of a given dosage at high dose rate produces more damage to a biologic system than exposure to same dosage given at low dose rate. Additionally, fractionation of the entire x-ray dose into multiple small doses imparts greater tumor destruction and permits better cellular repair of normal tissues [3]. The fractioned dose was based on the re-oxygenation, redistribution, recruitment, regeneration and repopulation of irradiated cells [10].
The results revealed that, at the coronal and middle thirds, the debridement efficacy of the experimental irrigants in Group II ‘radiotherapy group’ was significantly less than that of Group I ‘nonradiotherapy group’ with more remaining smear layer on root canal surfaces. This implies that changes created in the organic [11] and mineral tissues [12] by irradiation therapy might possibly increase the amount of the smear layer that consequently might feasibly influence the smear layer removal capability of the experimental irrigants.
Dentin is a hydrated biological composite composed of 70% inorganic material, 18% organic matrix and 12% water (wt.%). The structural composition of dentin includes oriented tubules surrounded by a highly mineralized cuff of peritubular dentin and an intertubuar matrix consisting of type I collagen fibrils reinforced with apatite [6,7]. It is well known that, the interaction of the organic matrix with apatite crystals of teeth results from the electrostatic binding of collagen carboxylate side chains and surface mineral phosphate groups via calcium ions. When the teeth are subjected to irradiation, the loss of acidic phosphate groups through the decarboxylation side chain, promoted by radiation, precedes the formation of new calcium ion bridge phosphate groups. Moreover, the mineral organic interaction is reduced, and the development of carbon dioxide may induce microcracks in the hydroxyapatite minerals, resulting in a roughened surface [6,7,13].
The ionizing radiations might have a detrimental effect on the hydrated collagen fibers by the action of free OH radicals [14].The formation of free radicals, from the reaction with the water molecules promotes the denaturing of the organic components of teeth [6,7]. Moreover, it was proposed that irradiation of protein leads to changes in their secondary and tertiary structures.
Furthermore, Cheung., et al. (1990) justified that irradiation was more damaging to organic components, mainly the collagen fibers [11].
At the apical third, the results revealed that there was no statistically significant difference between Group I and Group II in subgroup B and subgroup C. These findings came in agreement with the results obtained by [15] Hitomi., et al. (2008) who reported that the EDTA treated specimens exposed to Cobalt 60 (Co60) therapeutic gamma-irradiation exhibited more remaining smear layer than that not exposed to gamma-irradiation [10]. Yet the differences were statistically non-significant.
The irrigation of the canals in the second subgroup was done with 5.25% NaOCl then 17% EDTA as a final rinse applied for 5 minutes. The results revealed that EDTA treated samples in Group I showed a smear free and a clean surface. It could be related to the chelating action of EDTA, which acts upon the inorganic components of the smear layer and decalcify the peritubular and intertubular dentin leaving the collagen exposed. Subsequently, the use of NaOCl dissolves the collagen, leaving the entrance of the tubules more open and exposed [16].These findings indicate that a chelating agent should be used to remove the inorganic components which are in agreement with other studies [1,2].
Our results revealed that EDTA treated samples in Group I ‘nonradiotherapy group’ exhibited a clean surface free of debris with open dentinal tubules at the coronal and middle thirds with no statistically significant differences in the mean rank of smear layer scores between them. EDTA created areas of erosions which were observed more at the coronal third than at the middle. At the apical thirds of the canals EDTA seemed to be less effective and not all dentinal tubules were opened. These results are consistent with other studies [17-20] which demonstrated that cleaning was more effective on the coronal and middle thirds than on the apical third. It was attributed to the larger canal diameter in the coronal and middle thirds, allowing a better flow of the solution and a further improvement of its efficiency for smear layer removal. Also, it was probably because of insufficient volume and/or penetration of the solution into the apical portion of the canal during irrigation [19,21].
Our results contrasting with the results of other findings [1,22] which verified that the use of NaOCl and 17% EDTA removed the smear layer and promoted satisfactory cleaning of the coronal, middle and apical thirds to the same degree.
In the present study the irrigation of the canals in the third subgroup was done with 5.25% NaOCl then Biopure MTAD as a final rinse applied for 5 minutes. The results revealed that MTAD treated samples in Group I showed smear free surfaces and patent dentinal tubules. In addition, almost no dentinal erosions were observed over the different areas of the root canals. These results confirm the findings of previous investigations [23-36].
The efficacy of MTAD could be due to its ability to remove organic and inorganic substances from root canal surface. This was assisted by the presence of citric acid and the presence of a detergent Tween-80. Citric acid is a crystalline organic acid that could help in removal of smear layer [27].Tween 80 (polyoxyethylene sorbitan monooleate) is a detergent present in MTAD and is a nonionic surfactant. It could help in reducing the surface tension of the solution, thereby enhancing the flow and the wetting ability. Accordingly, more contact between the solution and the root canal surface could be obtained and the penetrating ability of the irrigating solutions into the root canal and the dentinal tubules might be increased [28].
BioPure MTAD also contains doxycycline hyclate in powder form; it is an isomer of tetracycline [2]. The effectiveness of doxycycline might be due to its low pH (pH of 2.15) thus could be able to act as a calcium chelator causing root surface demineralization. Additionally, its anti-collagenase activity and its ability to bind to dentin and to be released gradually over time might enhance removing organic and inorganic substances from the root surfaces [29].
5.25% NaOCl + MTAD treated samples in Group I exhibited a clean surface free of debris with patent dentinal tubules at the coronal and middle thirds with no statistically significant differences in the mean rank of smear layer scores between them. MTAD did not completely remove the smear layer at the apical third. This might be attributed to the decreased diameter of the canal till reach the least diameter at the apical third. In turn the amount of irrigating solution reaching the apical third is diminished [2].
The results showed that both 5.25% NaOCl+17% EDTA and 5.25% NaOCl + MTAD in Group I were equally effective in smear layer removal at the coronal and middle thirds this came in agreement with other investigations [2,24,30]. Also, both were inefficient in removing smear layer at the apical third. This came in agreement with others [23,31,32] who reported that EDTA and MTAD had equal capacities for the smear layer removal when used as a final rinse. Our results consistent with those obtained with some investigators [33,34] who found that MTAD and 17% EDTA removed the smear layer at the coronal and the middle thirds but did not have the ability to clean the apical area.
On the other hand, the results are in contradiction with those obtained by other studies [2,25,30] which testified that MTAD was significantly superior to EDTA in smear layer removal at the apical third.
Within the limitations of this in-vitro study, it could be concluded that: The irradiation dose (60 gray delivered in fractions of 2 Gy/day) used in this study had significantly reduced the debridement efficiency of the irrigating solutions utilized in this study at the coronal and middle thirds.
Both EDTA treated samples and MTAD treated samples in Group I were effective in smear layer removal; whereas saline treated samples did not efficiently remove the smear layer. Additionally, both EDTA and MTAD treated samples in Group I were equally effective in smear layer removal at the coronal and middle thirds. On the contrary, both were inefficient in removing smear layer at the apical third.
Special words of thanks are extended to Dr. Hany William Zaky, Lecturer (colleague) of Radiation Oncology, Head of Oncology Department; Ahmed Maher Teaching Hospital. I am deeply grateful for his acceptance to be a member of the practical and reading committee. Great thanks to Dr. Ahmad ABDOU, teaching assistant, faculty of oral and dental medicine, Modern University for Technology and Information. I am sincerely appreciative to him for performing the statistical analysis.
The study was partially funded by the national research center, Egypt. Other than that, authors deny any conflicts of interest in this study.
Copyright: © 2019 Shaimaa Rabea Mohamed., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.