P S Ojha, Vijaya Tripathi* and Pradeep Dwivedi
Nutrelis Agro Foods, Sector 63, Noida, India
*Corresponding Author: Vijaya Tripathi, Nutrelis Agro Foods, Sector 63, Noida, India.
Received: September 09, 2024; Published: October 16, 2024
Citation: Vijaya Tripathi., et al. “Antioxidant Capacity and Radical Scavenging Activities of Moringa oleifera". Acta Scientific Agriculture 8.11 (2024): 35-37.
Due to its numerous health benefits and potent antioxidant properties Moringa oleifera has attracted a lot of interest. This study assesses the antioxidant and radical scavenging properties of leaf extracts from Moringa oleifera, with a particular emphasis on the plant's bioactive components and their potential to mitigate oxidative stress. Its strong antioxidant activity may be attributed to the presence of phytochemicals such as flavonoids, phenolic acids, alkaloids, and vitamins (especially C and E). In vitro methods including the DPPH 2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay, the ABTS (2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid) assay, and the FRAP (Ferric Reducing Antioxidant Power) assay were employed to assess were employed to assess antioxidant activity. The findings showed that Moringa oleifera exhibited remarkable effects on free radical scavenging, with IC50 values antioxidants like tocopherol and ascorbic acid. At greater extract concentrations, the DPPH scavenging activity showed 70% inhibition rate moringa's ability to neutralize free radicals. Furthermore, there was a positive correlation observed between the antioxidant potency the extracts and their total phenolic content (TPC) and total flavonoid content (TFC), indicating that these chemicals are important contributors to the plant's overall antioxidant activity. These results demonstrate the potential of Moringa oleifera as a natural antioxidant source, positioning it as promising option for functional food products and nutraceuticals designed to reduce oxidative damage. The plant's capacity to counteract oxidative stress raises the possibility of its use in the prevention of chronic ailments like cancer, diabetes, and cardiovascular disease, all of which are known to be exacerbated by oxidative stress. Further research is imperative to elucidate its mechanism of action and potential therapeutic applications.
Keywords: Moringa oleifera; Oxidative Stress; Phytochemicals; Flavonoids; Phenolic Acids
Macrophomina phaseolina is a soil-borne phytopathogenic fungus having a wide host range of about 500 cultivated and wild plant species worldwide [1]. Important diseases caused by M. phaseolina include collar rot, damping off, charcoal rot, stem rot, root rot and seedling blight in economically important crops [2]. The plants affected with fungus exhibit necrotic lesions on different parts such as branches, peduncles and stems. A higher temperature and low moisture favour disease development [3]. The microsclerotia of the pathogen can survive on infected plant debris and soil for a long period, i.e., 2-15 years depending on the environmental conditions [4]. Microsclerotia are usually spherical, black and oblong. However, there is a great variation in their shape and size depending on the substrate, isolates and temperature [1]. M. phaseolina affects the plant by secreting an array of cell wall degrading enzymes which depolymerize the cell walls components such as cellulose, xylan, pectin, polygalacturonic acid and other proteins [5]. The most significant enzymes secreted by M. phaseolina are pectinases, xylanases, cellulases and proteases [6].
Cultural strategies such as crop rotation have been insufficient in controlling M. phaseolina. The use of chemical fungicides has been effective and a common practice in controlling M. phaseolina, but the rise of a resistant population has been reported throughout. Furthermore, there is an increasing concern over toxicity hazards from accidental exposures to the users, non-target organisms and potential environmental contaminations. Thus, there has been an increasing demand for chemical-free food production and the organic production system has become the fastest-growing sector of crop production.
Taking the aforementioned points into account, a research was conducted on chickpea plants infected with dry root rot to study their infection patterns and develop effective management practices for this disease.
The aim of the study was to investigate the infection patterns of dry root rot in chickpea plants and to devise efficient management strategies for this disease, considering the growing demand for organic food production and the rapid expansion of the organic agriculture sector.
The diseased roots of chickpea exhibiting the typical symptoms of dry root rot were collected from Agronomy farm, Anand Agricultural University, Anand during Rabi 2022-23; placed in brown paper bags and brought to the laboratory for microscopic examination and isolation purposes.
Visual and microscopic examination of typical dry root rot samples was carried out to confirm the presence of the pathogen. The typical symptoms of dry root rot on roots, shoots and leaves of chickpea under field conditions were visually observed and meticulously documented (Figure 1).

Figure 1: Symptoms of M. phaseolina on Chickpea.
The pathogen was isolated from root tissues of chickpea displaying characteristic symptoms of dry root rot symptoms. A standard tissue isolation procedure was followed for the isolation of the pathogen [7].
As shown in Figure (2) identification of pathogen-causing dry root rot of chickpea grown on PDA medium was examined visually as well as microscopically for cultural and morphological characteristics. The cultural characteristics including colony colour, colony topography, colony margin and colony diameter were recorded from the initiation of growth up to 10 days. The photomicrographs of these characteristics were also taken.
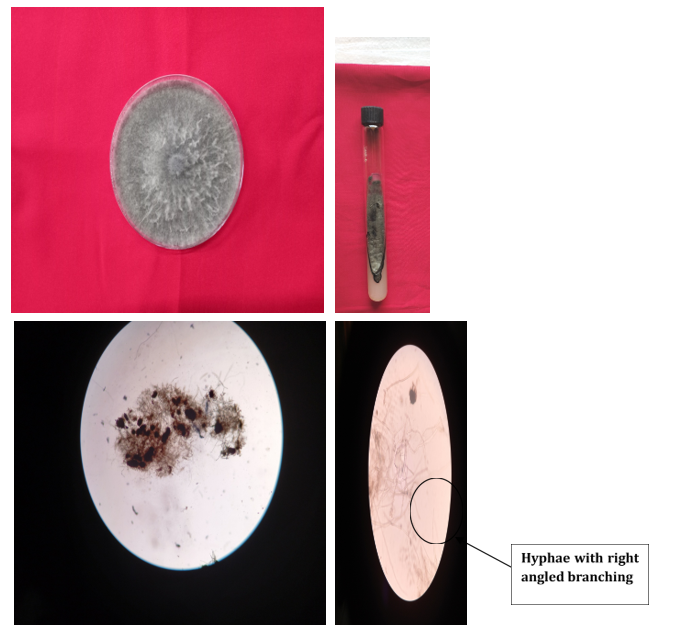
Figure 2: Morphological identification of M. phaseolina.
As illustrated in Figure (3) the pathogenicity of M. phaseolina was demonstrated through artificial inoculation of the pathogen following standard methods of inoculation (Koch’s postulates). Prior to the experiment, the soil and earthen pots underwent sterilization as previously described.
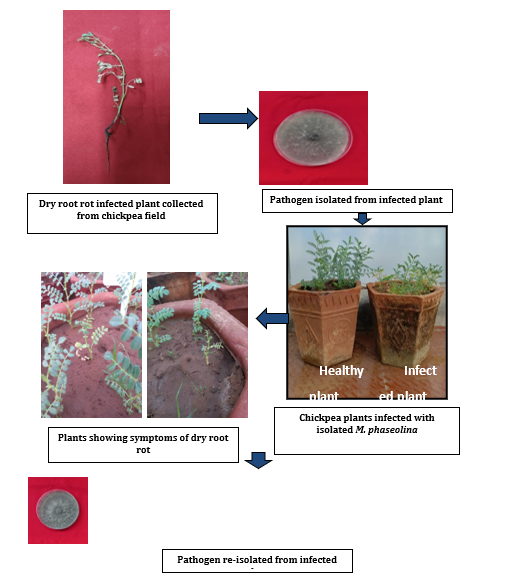
Figure 3: Pathogenicity of M. phaseolina on chickpea.
The sick pot technique developed by Nene., et al. (1981) [8] was employed to confirm the pathogenicity. Autoclaved soil was filled in 30 cm earthen pots at a rate of (2.5 kg/ pot) and inoculated with the pathogen (M. phaseolina) multiplied on 100 g of sand maize meal medium at the ratio of (9:1) in 250 ml conical flasks for 15 days inoculated at 25 ± 1 °C. The fungus grown on sand maize meal medium in flasks was added at a rate of 100 g/2 kg of soil. Control pots without inoculums was also maintained. Following a week of colonization in soil, seeds were planted in the pots. Rotted seedlings were examined post germination.
The fungus was subsequently re-isolated from the seedlings exhibiting rotting symptoms in these artificially inoculated pots using the tissue isolation method. The cultures obtained by re-isolation were transferred on PDA plates for comparison with the original culture and further investigations.
(Figure 3)
The examination of typical dry root rot (DRR) samples visually confirmed the presence of the pathogen. The disease starts to appear after 35-40 days of sowing. It is favoured by high temperatures and moisture stress conditions. Initial symptoms include scattered necrotic lesions on the roots, followed by rotting and withering of the lateral roots, accompanied by prematurely dried, straw-coloured leaves. The foliar symptoms begin with gradual yellowing from the base to the upper leaf and shoots develop a brownish colour. Diseased chickpea plants in the field show premature leaf yellowing and drying. Infected plants die prematurely due to progressive infection in roots, plant development stage and low soil moisture.
Similar observations were also observed by Sharma., et al. (2015) [9], Rai., et al. (2022) [10] and Mirchandani., et al. (2023) [11]. They observed that the dry root rot (DRR) symptoms are most commonly observed in chickpeas during the post-flowering stage which include drooping and chlorosis of petioles and leaflets. The leaves and stems of the affected plants are usually straw coloured and in some cases the lower leaves and stems are brown. The tap root becomes black due to decay and is devoid of the majority of lateral and finer roots.
Infected plants were examined for the isolation of pathogen using the standard agar plate method. Infected roots showing typical symptoms of DRR along with some healthy portions were aseptically transferred to sterilized PDA plates and incubated at room temperature. Fungal colonies emerging from the plates were transferred to fresh PDA plates using the hyphal tip method. In all investigations, the pathogen was preserved in a pure state of culture by periodically transferring it to PDA plates. The fungus colony on PDA expanded quite quickly, reaching a diameter of 90 mm at a temperature of 30 ± 1°C in just seven days.
The mycelial growth was fluffy, linear and coloured from dirty white to light grey at first. However, it eventually turned blackish grey and had brown to black microsclerotia. The microscopic examinations demonstrated that M. phaseolina hyphae were aerial, hyaline, light brown or dark brown, with thin walls and septa produced close to the branching and the hyphae branching were at a right angle with a constriction of hyphal branches at their origin. The mycelium's cells had a barrel form. The process of sclerotial development involved the aggregation of 50–200 individual hyphal cells, resulting in multicellular entities known as “microsclerotia”. The sclerotia varied in size and shape and ranged in colour from dark brown to black. Based on cultural and morphological characters, the pathogen was primarily identified as Macrophomina phaseolina (Tassi) Goid.
The pathogenicity of M. phaseolina was proved by artificial inoculation of the pathogen following standard methods of inoculation (Koch’s postulates). The disease was successfully produced in the sick pots inoculated with M. phaseolina 35 days after sowing whereas in control pots no disease was encountered. Symptoms in inoculated pots included rotting of primary roots followed by withering of lateral roots. Due to blockage in primary roots, the above-ground parts were devoid of water and minerals resulting in chlorosis of leaves and premature death of the plants. On the other hand, uninoculated plants showed no symptoms and remained healthy.
Infected plants were collected from artificially inoculated chickpea plants showing typical symptoms of DRR and reisolated separately on a PDA medium. The reisolation led to a pure culture that was similar to the originally isolated M. phaseolina. The pathogenicity of M. phaseolina has therefore been confirmed by artificial inoculation methods in accordance with Koch’s postulates.
As a result, the meticulous identification and confirmation of M. phaseolina as the causal agent of dry root rot in chickpea plants mark a significant milestone in agricultural research. These findings underscore the urgency of implementing targeted strategies to combat this destructive pathogen, safeguarding crop yields and ensuring food security. Moving forward, further exploration into innovative disease management approaches and the development of resistant crop varieties will be paramount in combating the challenges posed by M. phaseolina and preserving the health and vitality of agricultural ecosystems.
Copyright: © 2024 Manjari1., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.