Anam1,2, Kusum Yadav2, Pushpa Singh1* and RK Singh1
1Division of Plant Physiology and Biochemistry, ICAR- Indian Institute of Sugarcane Research, Lucknow, India
2Department of Biochemistry, University of Lucknow, India
*Corresponding Author: Pushpa Singh, Division of Plant Physiology and Biochemistry, ICAR- Indian Institute of Sugarcane Research, Lucknow, India.
Received: September 13, 2024; Published: September 26, 2024
Citation: Pushpa Singh., et al. “Navigating Sugarcane's Growth Matrix for Yield Maximization through Ethrel and GA3: Beyond Traditions". Acta Scientific Agriculture 8.10 (2024): 50-69.
Understanding the roles of hormones and enzymes in sucrose biosynthesis, metabolism, and accumulation is crucial in sugarcane. Sucrose phosphate synthase, sucrose synthase and invertase, along with auxin, ethylene abscisic acid, cytokinin and gibberellins are pivotal in regulating growth, sucrose accumulation and yield. Despite agronomical, physiological, biochemical and advancements in crop improvement, sugarcane yield remains low in various regions at critical growth junctions. Crop yield is constrained by poor germination (35-40%), low tillering and lesser number of millable canes (NMC). Such limitations also lead to sub-optimal sprouting, poor tillering, high tiller mortality and as result ratoon yield too decline. However, foliar application of growth hormones has mitigated these constraints by influencing biological processes, gene expression, and yield components. Ethrel and Gibberellin (GA3) are significant players in sugarcane agriculture, impacting cane and sugar harvest indices. This current article reviews roles of these chemicals in regulating sugarcane growth and development, provides insights into fundamental mechanisms and practical implications. By examining the effects of Ethrel and GA3 on overcoming limitations and enhancing obtainable yield potential (OYP) against theoretical yield potential (TYP, this article contributes to a deeper understanding of plant growth regulators utilization in sugarcane cultivation.
Keywords: Germination; Leaf Characteristics; Shoot Numbers; Canopy Coverage; Internodal Elongation; Biomass Accumulation; Stalk Length
Production of sugarcane (Saccharum officinarum L.) increased from 26 million hectares of cultivated land worldwide in 2008 to approximately 1.9 billion tonnes in 2023 [1]. It has a strong, fibrous stem that is jointed and produces between 40 to 70 metric ton dry weight ha-1 annually [1]. It goes through distinct stages during its growth cycle, namely germination, tillering, grand growth, and maturity [2]. The germination stage refers to duration from planting up to protrusion of buds from the setts and lasts for 45 days [3,4]. The tillering stage is duration of 120 days, wherein multiple tillers with nodes and internodes are produced and governs the net productivity [5]. The grand growth stage ranges between 70-170 days and is a period of actual stalk elongation and internodal growth [5]. The leaves located on every internode serve as primary organs for photosynthesis while sections of the stem between the nodes with sucrose storing parenchyma cells and vascular tissues functions as sink organs [5]. The maturity/ripening stages is of about 90 days, where sucrose accumulation occurs [6]. The key reasons identified for yield stagnancy are delayed germination, slow pace of early phase of development, poor tillering, limited internodal elongation and higher dry matter losses during the crop cycle [4]. Delay in germination reduces the growth period of sugarcane crop cycle [4]. The canopy coverage and development of leaf area index (LAI) leads to poor accumulation of dry matter [7]. The reduced leaf area index (LAI), low canopy coverage, rate of leaf area expansion, leaf number, shoot population, root density and total plant dry weight changes lead to poor accumulation of photosynthates [7]. Photosynthesis process is governed by water use and nitrogen use efficiency, which is an index of physiological efficiency. Improved production efficiency strengthens photosynthetic rate of the plant, transpiration rate, stomatal conductance, and internal CO2 concentration and dry matter production [5,8,9].
As growth phase is of 360 days, the crop experiences low temperature, high temperature, drought and rainy season [2,5]. Its germination phase often coincides with low and high temperatures while the rest of the critical stages experience around 10-13°C above the ideal growth conditions. Such changes have exhibited severe loss in soil, sett moisture content and distinct array of cellular and metabolic reactions [10]. This degrades proteins and inactivates enzymes in addition to damaging membranes.
This process also causes pigment fading and DNA strand disruption, leading to cellular moisture deficit and hindering tiller formation [11]. The cellular and metabolic changes have been reported to have imposed severe limitation on early germination process, establishment of seedlings and duration of crop growth [4,12]. Higher temperatures have been reported to diminish CO2 intake, decrease photosynthetic rate, reduce dry matter partitioning, thus impede plant's capacity for growth [4]. High temperatures have also affected the synchronism existing between mother shoot and tillers, movement of metabolic by products and nutrients adversely resulting in a reduction in number of millable canes. The tiller numbers thus are reduced at tillering phase. Reduced tiller numbers affect the productivity adversely as tillers per plant determine the quantity of millable canes at harvest stage. The leaf development, canopy coverage, light interception level, stalk elongation, leaf area expansion and cumulative growth rate during tillering and grand growth phases too are impacted adversely and as a result, the adverse temperatures have restricted the tiller formation [5]. It lowers the availability of reducing sugars, simultaneously decreasing the activity of acid invertase, while raising the levels of indoleacetic acid (IAA) and phenols. The accumulation of these compounds in sugarcane buds in their natural environment results in bud dormancy [4].
Plant growth regulators are a wide range of organic compounds produced by plants for their own growth and development of their defense system [13]. The compounds are produced in small amounts and are primarily used on-site [12] or applied exogenously for their onsite usage [14,15]. As the impacts on developmental processes are dose dependent, their exogenous application has produced remarkable results on plant growth and stem elongation [16]. Exogenous application is conducted through foliar spray on plants and soil, in vivo injection, pre-planting treatment, drenching, seed priming, capillary wick techniques, and the use of pastes are commonly employed [17]. Ethrel (ethylene releasing compound) and GA3 are plant growth regulators which have been used in several crops and sugarcane since decades. In light of above, the current paper reviews the advantages of applying Ethrel (ethylene releasing compound) and GA3 exogenously at critical growth junctures in sugarcane crop cycle.
Sugarcane growth is delayed due to poor germination (merely 30-33 %) and slow pace of growth in early phase of development during the crop cycle. The slow and delayed bud germination limits the early vigor of the cane crop (Figure 1). The time taken in germination varies from 40 to 45 days after planting which reduces the growth duration of 360 days, 45 days are consumed by the germination phase only. As a result, the canopy coverage and leaf characteristics development are quite slow and this impacts the dry matter accumulation adversely. The trend of germination remains similar in the ratoon crops too. Irregular germination in both timing and spacing, with around 10-15% of bud setts failing to sprout, results in a 20-25% gap in the crop stand [4].
Exogenous ethylene has led to improvement in germination in numerous species [18]. Exogenous ethylene has led to stimulation of germination in external environmental conditions such as extreme high temperature, osmotic stress, salinity and hypoxia [19]. Since, ethylene is gaseous in state, it is now available commercially in liquid form to users as Ethrel (Ethephon, 2-chloroethanephophonic acid). Ethrel releases ethylene, breaking seed dormancy and promoting increased germination in seeds with seed coat-imposed dormancy across various species [20].
Germination takes place in soil where seeds are exposed to various environmental factors such as temperature, moisture, oxygen, and light [21]. Germination consists of three phases: Phase I, Phase II, and Phase III. In Phase I, germination begins with imbibition, which activates respiratory metabolism as well as transcriptional and translational activities. Phase II halts water uptake and focuses on reserve mobilization. Phase III is marked by the protrusion of the radicle, signifying the visible onset of seedling growth [22,23]. Ethylene (C2H4) plays a key role in controlling germination and dormancy across many species through a complex network of hormonal signaling pathways [18]. It is produced in the seeds immediately after the onset of imbibition and increases with germination. The ethylene biosynthesis pathway in seeds and setts closely resembles the process observed in other plant organs (Figure 2). S-adenosyl methionine (S-AdoMet) and ACC are the primary intermediates [24]. S-AdoMet, produced from methionine by S-AdoMet synthetase (SAM synthetase), is subsequently converted to ACC by the enzyme ACC synthase (S-adenosyl-L-methionine methylthioadenosine-lyase, ACS). S-AdoMet is the precursor of the biosynthesis of polyamines and plays a vital role in germination of seeds [24]. ACC produces ethylene by ACC oxidase (ACO) along with CO2 and HCN (Figure 2). 5 -methylthioadenosine (MTA) is a by-product during the reaction process [24]. It has been well documented that ethylene regulates the ACO expression. It has also been reported that increased ethylene production during germination is associated with an increase in ACO activity, as well as a progressive accumulation of ACS and ACO transcripts [18]. Both ACS and ACO are encoded by a multigene family while the regulation of ACS and ACO genes differ among each other [24].
Ethylene plays a crucial role in various developmental processes and in the response to both biotic and abiotic stresses in plants [24]. ACS is a key enzyme that regulate ethylene production in most plants, under abiotic and biotic stresses [24]. It is also reported that ACO activity also plays a fundamental role in seed germination [18]. Ethylene acts on the cell surface where there exist five membrane-localized ethylene receptors, ethylene resistant 1 (ETR1), ETR2, ethylene response sensor 1 (ERS1), ERS2, and ethylene insensitive 4 (EIN4) [24]. ETR1 and ERS1 have three trans-membrane domains at the N-terminus and a histidine kinase domain at the C-terminus. Conversely, ETR2, EIN4, and ERS2 possess four trans-membrane regions and a serine-threonine kinase domain at the C-terminus [24,25]. When ethylene binds to its receptors, it leads to the inactivation of the CTR1 (constitutive triple response 1) protein kinase. This inactivation triggers a kinase cascade that activates EIN2 and its associated transcription factors in the nucleus. EIN3, EILs (EIN3-like proteins), and ethylene response element binding proteins (EREBPs) or ethylene responsive factors (ERFs) then promote the transcription of genes responsive to ethylene [24]. (Figure 2).
Sugarcane buds first germinate to produce primary shoots. As these primary shoots develop, they form stools, which are clumps of shoots. Subsequently, secondary shoots emerge from the basal buds of these stool [26]. Sugarcane exhibits sympodial growth, which is supported by the replacement of adventitious buds. Each axillary bud contains a band of root, scar, and wax located at the node. These buds are embryonic shoots with the potential to develop, a process known as 'germination.' After germination, primary and secondary shoots emerge, leading to the formation of tertiary shoots. Auxins play a role in the development of sett roots. The shoot roots that emerge from the lower part of the developing shoots gradually replace the sett roots within 45-60 days. Sugarcane is propagated vegetatively from stalk pieces and is cultivated through successive ratooning cycles [27]. These buds may fail to germinate due to factors such as apical dominance inhibition, injury, stem desiccation, excess water, inadequate nutrition, or infection by organisms leading to sett and root decay [10]. When an intact sugarcane plant experiences complete or partial mitotic activity retardation by the apical bud, the lateral buds' meristems stay dormant. This is caused indirectly by auxin, which is transported basipetally. Secondary messengers, including ethylene and abscisic acid, are thought to transmit inhibitory signals to lateral buds. In sugarcane, this triggers a complex series of physiological and biochemical events that lead to bud break. Plant hormones, the activity of specific enzymes, and alterations in food metabolites all contribute to this complexity. Therefore, the functions of individual hormones indicate to plant regulators of growth that can be used to boost bud germination and expansion that comes next.
The germination, growth, and sugar yield of sugarcane setts and ratoon stubbles have all improved with the use of plant growth regulators [28]. Through promoting IAA metabolism and preventing IAA's polar transport, ethylene contributes to the induction of adventitious roots. Ethylene's effect on lateral buds is similar to decapitation, as it stimulates peroxidase activity, which degrades auxin. and restricts the main shoot, side shoots, and tillers. Ethrel (2-chloroethanephophonic acid) is an ethylene releasing compound used for dipping of setts for improved bud germination. The germination of sugarcane varieties was significantly enhanced by ethylene at 120, 240, 360, and 480 parts per million [29].
Three-budded setts of sugarcane are cut from main cane stalks. Before planting, setts are treated with Ethrel at a concentration of 100 ppm (Table 1,2). The setts are soaked overnight and removed the next morning for planting. Before planting in the furrows, they are rinsed in Bavastine at a concentration of 2 g/L. (Figure 3). Usage of Ethrel on cane setts suggests that ethylene may act on the cell surface, where membrane-localized ethylene receptors are present, existed (ETR1, ETR2, ERS1, ERS2 and EIN4). Binding of ethylene to its receptors likely results in the inactivation of CTR1 protein kinase. This inactivation activates a kinase cascade that controls EIN2 and its associated transcription factors in the nucleus. EIN3, EILs, and EREBPs/ERFs then promote the transcription of genes responsive to ethylene [24]. (Figure 2). These suggested that due to these alterations, Ethrel induced faster heterotrophic to autotrophic transitions for establishing high initial plant population [4]. Setts were soaked overnight in 100 ppm Ethrel before planting. The protruded buds have higher bud moisture content, dry weight and growth rate, that were maximum at 20 DAP itself. The increase in dry weight and growth rates were commensurate with bud moisture. Maximum settling/shoot population was obtained (55,000 shoots ha-1) with Ethrel at 20 DAP (Figure 4 and 5). Later at 45 DAP also, bud moisture, bud dry weight, RGR and settlings population were highest with Ethrel treated setts against untreated setts (Figure 4). The settling population was found to have reached a maximum of 65,000 shoots ha-1 with Ethrel treated setts against 42,450 in untreated buds at 45 DAP (Figure 4 and 5). This indicated that exogenously applied Ethrel treated setts induced earliness in germination and completed the process at 20 DAP. Thus, it provided an initial lead of about 25 days for crop growth [30].

Table 1: Modus operandi for exogenous application of Ethrel in sugarcane under field conditions
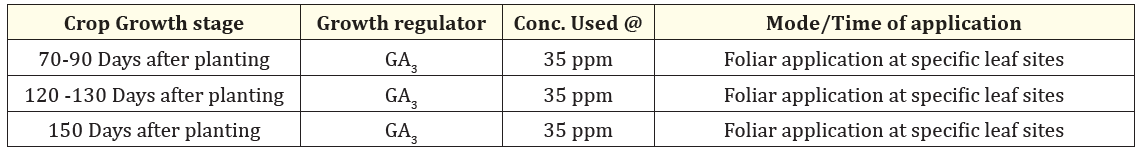
Table 2: Modus operandi for exogenous foliar application of GA3 in sugarcane under field conditions
The impacts of Ethrel led to fourfold increase in germination % at 20 DAP, which was commensurate with high acid invertase (AI) activity that led to increase in reducing sugar content and decrease in sucrose levels [4]. In addition, a fourfold increment in reducing sugars and twofold decline in sucrose contents was recorded with Ethrel at 20 and 45 days after planting respectively, against untreated setts respectively [4]. Increased reducing sugar and decreased sucrose caused by higher AI activities led to enhanced growth of buds and emergence of settlings at 20 DAP. NR activity in vivo, SOD and IAAO activities in Ethrel setts were elevated at 20 and 45 DAP respectively. Increased NR activity in vivo, IAAO and SOD activities supported the faster sink to source transition and growth of bud. There was a seven-fold increase in NR activity in vivo and fivefold increase in IAAO activity at 20 and 45 DAP respectively, in Ethrel treated setts. Also, a sevenfold increase in SOD activity was recorded in Ethrel treated setts at 20 and 45 DAP. Increase in IAAO activity resulted in decrease in IAA contents at 20 and 45 DAP in Ethrel treated setts. The fourfold and twofold decrease in IAA contents and total phenolic contents was obtained in Ethrel setts respectively at 20 and 45 DAP against untreated setts (Figure 4). Ethrel induced enzymatic and metabolite changes led to establishment of initial population of 55,000 settlings ha-1 at 20 DAP. Thus, it led to gain of 20 days in initial crop growth period due to early sprouting and rapid flush of shoots and leaves on young settlings [4].
Gibberellins, are complex natural biomolecules with a tetracyclic carbon skeletal structure [31]. They were isolated from the fungus Gibberella fujikuroi and later on it was reported from plants too. They are known to regulate multiple physiological processes, such as stem elongation, seed germination, flowering, and fruit development [31]. It regulates the synthesis of auxin, transporters and increases stress tolerance for organ formation and gravitropism [32]. Exogenous application of GA3 has been reported to cause an etiolated phenotype, with thinner leaves, a larger leaf area, and lower chlorophyll content [33]. Its application has generated elongated, narrow leaves and extended internodes in Lactuca sativa L. and Eruca sativa L [34].
GA3 has gained worldwide attention due to their successful use in agriculture, nurseries, horticulture, tissue culture, tea gardens, etc [35]. Though there is great diversity in response towards GA applications in plants, yet the most noticeable impact was observed in plants. The ratio of carbon and total dry weight in these plants has been reportedly increased suggesting involvement of GA in increasing the rate of carbon fixaton during photosynthesis. The first successful application of gibberellic acid was realized through commercial production of Thompson seedless grapes in California in 1962 and through production of world famous Clementine Mandarin oranges [13]. As plants produce low amount of GA, they are applied exogenously. They are commercially available for usage by farmers.
The biosynthesis of gibberellins in plants occurs primarily in the young growing shoots, roots, and developing seeds [36,37]. It involves several enzymatic steps and formation of intermediate compounds [38]. The biosynthesis begins with the formation of terpenoid precursors, such as geranyl geranyl diphosphate (GGDP), derived from the mevalonic acid pathway (Figure 6). It is then converted into ent-kaurene, key intermediate in gibberellin biosynthesis. This conversion involves a series of enzymatic reactions catalyzed by enzymes, ent-copalyl diphosphate synthase (CPS) and ent-kaurene synthase (KS). ent-Kaurene then undergoes modifications to form active gibberellins. This conversion includes a continued series of oxidation steps and structural modifications catalyzed by various enzymes, including cytochrome P450 mono oxygenases at the endoplasmic reticulum and 2-oxoglutarate-dependent dioxygenases in the cytosol of the cell [38]. The final steps of gibberellin synthesis involve further oxidation and rearrangement of the modified ent-kaurene compounds, resulting in the production of bioactive gibberellins, such as GA1, GA3, and GA4 [36]. The biosynthesis of gibberellins is tightly regulated by environmental cues, hormone interactions, and developmental stages [39]. It involves the activity of the enzymes involved in the synthesis pathway [39]. Once synthesized, gibberellins are transported within the plant through the vascular system [40]. It can move upward or downward in the plant, influencing various tissues and organs, including shoots, roots, and developing seeds and its transport involves both passive diffusion and active transport mechanisms [41].
Exogenous application of GA induces transverse reorientation of microtubules in cell wall of dwarf pea plants that results in longitudinal expansion changing dwarf mutants to tall ones [42]. The basic mechanism of GA mediated elongations through exogenous GA3 application leads to active transport of solutes into the vacuoles present in plant cells and causes passive influx of water and generates turgor pressure (Figure 7). This turgor pressure creates osmotic imbalance between the intracellular and extra cellular fluids and provides the driving force for cell expansion. The cellulose-hemicellulose network and matrix polysaccharides defines the shape of differentiated cells and determines the direction of cell elongation. Turgor pressure being a non-directional force can result in multidirectional expansion, however this expansion is channelled in proper direction by transverse arrangement of inelastic cellulose microfibrils. Cell walls mostly expand in direction perpendicular to the orientation of cellulose microfibrils. Cellulose microfibrils are invariably found to be oriented transversely to the elongation axis and loosened longitudinally during cell elongation. This microfibril orientation is regulated in proper direction (transverse orientation) by GA3 to produce an elongated shoot- root axis [43]. GA3 helps in maintaining the transverse orientation of cellulose microfibrils in cells, thereby extending the elongation zone of the internode [44]. The activity of xyloglucan endo Transglycosylase (XET) that involved in cell expansion and organ growth is increase by GA3. It induces cell division as well as vacuolation in epidermis, cortex and pith in some plants resulting in rapid growth and elongation.
Expansin is cell wall loosening enzyme which is actively involved in elongation of growing region of the internodes [41]. It helps in loosening of the cell walls by acting as a kind of molecular grease and binds at the interface between cellulose microfibrils and matrix polysaccharides. It induce extension by reversibly disrupting the non-covalent bonds probably by catalysing the disruption of H- bonds within polymeric network [44]. This facilitates the turgor driven slippage between microfibrils and other components of the cell wall allowing controlled relaxation of the wall needed for cell elongation (Figure 7). GA3 induced elongation is regulated by feedback mechanism whereby the action of the active GAs results in the production of transcriptional repressor that limits the expression of GA biosynthetic enzymes [31]. Besides elongations, cellular divisions also contribute to growth, however since meristematic cells are small, the divisions occurring in apical meristems, do not add greatly to the mass or length of plant and cell elongations remain the major role players in plant [41].
GA signalling involves a balance between suppressing genes related to GA biosynthesis and boosting the formation of GA receptors and enzymes that are responsible for breakdown of active GAs [45]. DELLA proteins inhibits the growth while activation of GA promotes growth [46]. DELLAs belong to super family of transcription factors unique to plant kingdom known as GRAS proteins. They have conserved C- terminal motif but divergent N terminal is named after a conserved motif (Asp-Glu-Leu-Leu-Ala) at their N termini, which is absent in other GRAS members. C terminal domain of DELLA proteins is involved in repressive action whereas N terminal domain is involved in perception of GA signals by DELLA proteins [47]. DELLA repressor proteins, GA receptors, and F-box proteins are the crucial players in controlling the stability of DELLA proteins. DELLA proteins are degraded in presence of GA and the prefoldin complex remains in the cytoplasm thereby producing active tubulin subunits [48]. DELLAs repress all forms of GA responses such as growth and all other known GA-dependent processes [45]. Initially, GA signal is perceived by the GA receptor GID1, a soluble protein that is localized to both cytoplasm and the nucleus (Figure 8). Binding of GA and C-terminal domain of GID1 receptor creates a conformational change as well as hydrophobic surfaces that enables recognition of DELLA proteins by G1D1 for binding. In GA-mediated plant responses, the role of DELLA proteins has been reported in various crops [47,49,50]. Post formation, GID1-GA-DELLA complex is recognized by F-box component of Skp1-cullin-F-box (SCF) ubiquitin ligase for polyubiquitylation, followed by its degradation through the 26S proteasome (Figure 8) [51].
Nuclear DELLA protein’s removal results in massive alteration in gene expression and culminates in cell elongation [51]. In absence of GA, DELLA protein acting in their normal mode of action will interact with the transcription factors or transcription regulators and changes them to inactive complexes which are no longer able to bind to the DNA molecule or transcription factors, respectively and thus unable to execute their normal action [52]. This molecular mechanism plays a critical role in multiple stages of plant physiology, including embryo development, triggering seed germination, root formation, leaf growth, stem elongation, the process of flowering, seed production, trichome formation, and pollen maturation [53,54]. In addition to regulating plant morphogenesis, gibberellins (GAs) influence microtubule orientation by facilitating the physical interaction between nuclear-localized DELLA proteins and the prefoldin complex, which acts as a co-chaperone necessary for proper tubulin folding [55]. Gibberellins (GAs) promote growth by mediating the proteasome-dependent degradation of DELLA proteins. Additionally, active GAs increase cell wall elasticity through the action of the enzyme XET (xyloglucan endotransglycosylase), which plays a key role in reorganizing the molecular structure of the plant cell wall's matrix [56]. Gibberellins (GAs) have been found to regulate the cell cycle by increasing the expression of specific kinases that are crucial for the cyclin CDC2 and M-phase cyclins, both of which are vital for activating mitosis [57]. These molecular processes drive the actions of gibberellins (GAs) in diverse plant species, emphasizing their critical role in regulating plant growth and development.
In sugarcane (Saccharum spp. hybrids), lower levels of sunlight (250 Langleys per day) and cooler temperatures, averaging 23°C daily, generally lead to a reduction in stalk length, fresh weight, dry weight, and sucrose content. Foliar application of GA3 plays an important role in sugarcane growth, especially in elongation of internodes that led to an increase in sucrose accumulation in stalks and thereby increases sucrose yield and number of cane stalks ha-1 [4,6,58,59]. Gibberellins are used to increase sugarcane yields has been reported since several decades [60-62]. An exogenous application of combination of Ethrel and GA3 at all the growth stages of sugarcane crop cycle has shown tremendous potential to increase the cane and sugar harvest index. It optimized shoot population by improving physiological efficiency through reducing the lag in emergence, improvement in germination percent and improve growth in early phase, synchronize the tillering and reduce the tiller mortality, thus improving tiller numbers and manipulated source and sink organs for diversion of photosynthates towards enhancing cane weight and sucrose content (Figure 1).
To prepare the GA3 solution, 0.5 cm³ of ethanol is used as a solvent. The solution is then diluted with distilled water to a concentration of 100 mM. It is applied to the plants using a knapsack sprayer, with 5 mL of the solution per plant, between 8 and 9 AM. The GA3 treatment is administered at 90, 120, and 150 days after planting (Figure 2). The volume of water used for the GA3 solution depends on the number of plants in each row.
After germination with exogenous application of Ethrel, GA3 was applied through foliar spray at all the critical growth stages (90,120,150 DAP). Foliar applications of GA3 at 90, 120, and 150 days after planting significantly enhanced various growth metrics, including the number of leaves, total leaf area, leaf area index, leaf area duration, biomass duration, leaf area ratio, and net assimilation rates at 180 and 270 days after planting, especially in the Ethrel-treated setts. (Figure 9). The applications led to highest foliage numbers at 180 DAP in Ethrel treated setts. There was sevenfold increase in leaf area index in Ethrel treated setts with GA3 at 180 DAP. It led fivefold and twofold increase in leaf area and leaf area index in Ethrel treated setts with GA3 application at 180 DAP. Duration of leaf area (LAD), ratio of leaf area (LAR) and period of biomass accumulation were elevated by four, five and seven-fold respectively at 180 DAP. At 270 DAP, despite a twofold decrease of leaf area, leaf area index, duration of leaf area and ratio of leaf area were maximum. Duration of Biomass accumulation (gd*103) increased by fivefold and six-fold at 180 and 270 DAP. The net assimilation rate, which measures the daily increase in biomass per unit of leaf area, was at its highest in Ethrel treated setts with GA3 application at 180 and 270 DAP. Architectural modifications led to quicker transitions from heterotrophic to autotrophic growth at the planting stage (February). This resulted in a high initial plant population at 45 days after planting, followed by the development of a more efficient canopy with increased source activity and enhanced sink development both above and below ground by 60 days after planting. Adjustments in leaf angle also improved CO2 utilization and radiation use efficiency (RUE) (Figure 9). The application of GA3 resulted in a more optimized canopy structure and better distribution of dry matter. The increased angle of leaf orientation reduced shading between leaves on the stalk, allowing the lower leaves to capture more light. Additionally, GA3 stimulated the growth of roots with a steep angle (30°), significantly increasing root weight and enhancing root hair development, which supported the nutrient needs of the larger shoot population. As a result, improvements were observed in net assimilation rates (0.65 cm² per day), ratio of leaf area (16 cm² per gram), and duration of leaf area (55 × 10⁴ cm² days), leading to greater internodal counts, lengths, and weights [4].
During the grand growth and harvest stages, the combination of Ethrel and GA3 treatment in plants resulted in a maximum of 6.73 lakh shoots per hectare and a number of mature culms (NMC) of 3.01 lakh per hectare. In comparison, the untreated plants achieved a maximum of 4.59 lakh shoots per hectare and an NMC of 1.32 lakh per hectare. (Figure 10). Increase in number of millable cane /clumps was recorded in plant as well as ratoon cane (Figure 11). The Ethrel-soaked setts treated with GA3 showed a notable increase in the average number of internodes, their length, and weight compared to the untreated setts.
A fivefold increase was recorded in mean internodal number per stalk at 270 days after planting. At 180 and 270 days after planting, internodal lengths increased six fold respectively. Mean internodal weight was maximum at 180 DAP and increased by twelvefold at 270 days after planting (Figure 11). The applications led to increase in shoot population, stalk length, stalk and root dry weights (Figure 12, 13 and 14). At 180 and 270 days after planting, the shoot numbers increased twofold and sixfold, respectively. Additionally, there was a fivefold and sevenfold rise in shoot numbers at these time points (Figure 12). Minimum shoot numbers were recorded in untreated setts without GA3 application. Maximum increase in stalk length and stalk dry weight at 180 and 270 days after planting were recorded with combined application of Ethrel and GA3. Stalk lengths increased by fourfold in Ethrel treated setts against untreated setts with GA3 applications at 180 and 270 days after planting, respectively (Figure 13). A significant sevenfold and eightfold increase were recorded in stalk dry weight in Ethrel soaked setts with GA3 application at 180 and 270 days after planting. Maximum dry matter content and root weights were recorded in Ethrel soaked setts against untreated setts with GA3 application at 180 and 270 days after planting (Figure 14). At both the stages, a threefold increase was recorded in root weights (Figure 14). The dry matter content, Brix% and purity of cane juice was higher in Ethrel treated setts against untreated setts with GA3 applications at 270 days after planting (Table 3). At the grand growth and harvest stages, the combination of Ethrel and GA3 achieved a maximum of 5.37 lakh shoots per hectare In contrast, the control group had 2.13 lakh shoots per hectare. Additionally, there was a recorded increase in the number of millable canes and clumps in both plant and ratoon crops (Figure 12).
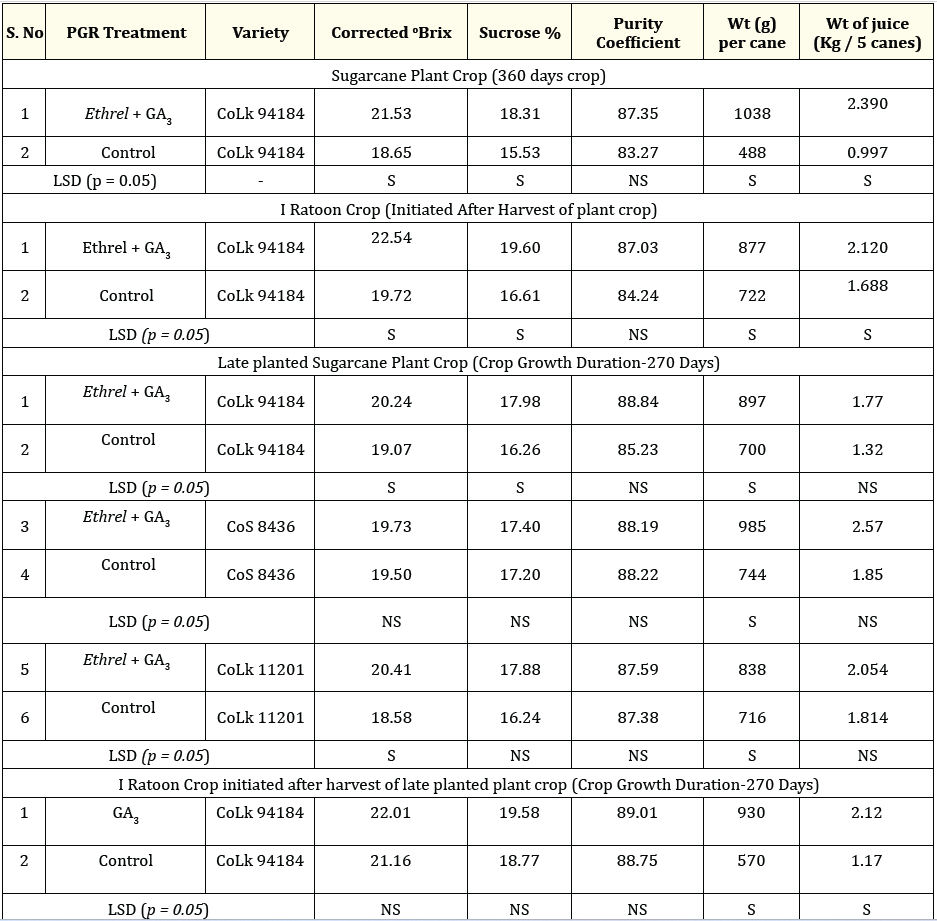
Table 3. Exogenous application of Ethrel + GA3 - Impact on growth attributes and juice quality in sugarcane plant and ratoon crops.
Values are mean of three replicates; * F interaction analysis for Corrected oBrix, Sucrose %, Purity Coefficient, Wt (g) per cane and Wt of
juice (Kg / 5 canes), LSD (p = 0.05); Least Significant difference, S; Significant difference, NS; Non significant difference
Foliar applications of Ethrel @ 100 ppm at 60 days after planting and GA3 at 90, 120, and 150 days after planting in the first ratoon crop led to a higher sprouting percentage, a decrease in tiller cessation, and a denser tiller population with enhanced stalk elongation rates (Figure 15). This resulted in a 66.5 tons per hectare increase in cane yield compared to untreated plants. Additionally, the in situ decomposition of sugarcane trash, applied at 12 tons per hectare after the harvest and treated with PUSA compost inoculant at 300 grams per ton of trash, led to a maximum of 5.37 lakh shoots per hectare, reduced tiller mortality to 54.5%, and maintained the number of mature culms and cane yield at 3.06 lakh per hectare and 183.2 tons per hectare (with an average cane weight of 598 grams), respectively. In comparison, the untreated plants had a maximum of 2.13 lakh shoots per hectare, 66.7% tiller mortality, 1.53 lakh mature culms per hectare, and a yield of 99.8 tons per hectare (with an average cane weight of 501 grams). (Table 4). The in situ decomposition of trash combined with foliar application of GA3 results in an increase in ratoon cane yield by approximately 16.9 tons per hectare [30].
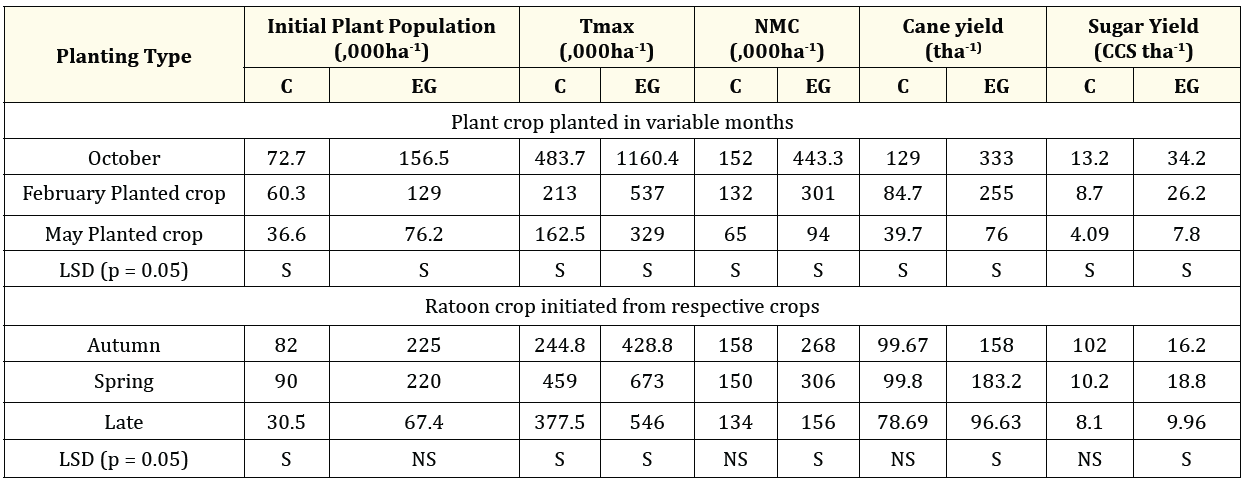
Table 4. Exogenous application of Ethrel + GA3- Impact on initial plant population, Tmax cane and sugar yield.
Values are mean of three replicates; * F interaction analysis for initial plant population, Tmax, NMC, cane yield, sugar yield, LSD (p=0.05);
Least Significant difference, S; Significant difference, NS; Non significant difference C – Control; EG - Ethrel + GA3.
Germination, development and accumulation of dry matter in both sugarcane plants and ratoon crops were enhanced by combined exogenous application of Ethrel @100 ppm and GA3 @35 ppm at critical growth phases under field conditions. Ethrel led to faster rate and enhanced germination in both plant and ratoon crops. This was due to improved ethylene pool in buds that led to activation of membranes for improved rate of imbibition, reserve mobilisation and faster radical protrusion of the buds on the vegetative setts. Ethrel application saves about 20-25 days from 45 days of the germination phase. As most of the buds germinated, a gain was obtained in the number of shoots at an early stage (at about 90 DAP). Ethrel led to improvement in leaf characteristics, increase in number of shoots, improved photosynthetic process, canopy coverage and robust root system with better nitrogen and water use efficiency. GA3 application influenced the cell membrane permeability that facilitated mineral nutrition, uptake and transport of photosynthates which led to improved biomass accumulation. GA3 led to marked changes in morphological traits and promoted the biomass accumulation towards leaves, initially and later towards the stalks or cane stems. Inducing cell division and elongation, Ethrel along with GA3 increased the height of the plant and enhanced the source and sink organs. The high density of stalks in a restricted ground area with Ethrel and GA3 was attributed to the formation of upright canopies and strong root systems.
This led to architectural alterations causing threefold increase in cane yield of plant and two fold increase in ratoon crop. Ethrel + GA3 application across the crop cycle merely involved an additional input cost of Rs. 8,500 ha-1. However qualitative and quantitative estimate of benefits to beneficiaries/stakeholders indicated that combined exogenous application offered an additional return of about Rs 60,000 -65,000 ha-1. Exogenous application of Ethrel and GA3 at key stages of the crop growth cycle can thus be recommended to farmers and commercial growers as a technique for promoting sugarcane growth and yield.
We thank the Director, ICAR-Indian Institute of Sugarcane Research, Lucknow, India, for his constant encouragement. We extend our thanks to the staff of the Division of Plant Physiology and Biochemistry for their continuous help during analysis and writing.
The authors received no specific funding for this work.
The authors report that there are no competing interests to declare.
Data will be available on request.
Copyright: © 2024 Pushpa Singh., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.