Hosna Elshony1*, Abdelrahman Idris2, Abdulaziz Al-Ghmadi2, Rakan Almuhanna2, Waleed Amsaib M Ahmed3
1Department of Neuropsychiatry, Faculty of Medicine, Menoufiya University, Egypt
2Department of Neurology/Internal Medicine, Security Forces Hospital, Makkah, Saudi Arabia
3Department of Infectious Diseases/Internal Medicine, Security Forces Hospital, Makkah, Saudi Arabia
*Corresponding Author: Hosna Elshony, Department of Neuropsychiatry, Faculty of Medicine, Menoufiya University, Egypt.
Received: July 18, 2024; Published: September 17, 2024
Citation: Hosna Elshony., et al. “Ictal Vomiting as an Unusual Presentation of Herpes Simplex Encephalitis - Pathophysiological and Therapeutic Perspectives". Acta Scientific Clinical Case Reports 8.10 (2024):21-27.
Introduction: Herpes Simplex Encephalitis (HSE) is a severe neurological infection that is often difficult to diagnose due to its varied clinical presentations. Ictal vomiting, a rare manifestation of seizures, is believed to originate from the anterior temporal or insular lobes, regions notably involved in HSV encephalitis.
Case Presentation: This case report describes a sixty-year-old female with diabetes, hypothyroidism, and hypertension, who presented with fever, vomiting. Initially, the patient's diagnosis was elusive despite hospitalization, and her condition deteriorated, exhibiting behavioral changes, cognitive decline, and focal seizures. Neuroimaging eventually revealed typical findings, confirming non-hemorrhagic herpetic encephalitis. Although treated with antiviral and antiepileptic medications, persistent vomiting led to further investigations. EEG identified infrequent right temporal sharp waves, resulting in a diagnosis of "ictus emeticus”.
Conclusion: This case of Herpes Simplex Encephalitis (HSE) highlights the diverse clinical spectrum and management challenges of the condition. The patient's unusual presentation underscores the importance of considering HSE in patients with fever and unexplained persistent vomiting for early diagnosis and better prognosis. Diagnostic tools, including neuroimaging, cerebrospinal fluid analysis, and electroencephalography, confirmed HSE involvement in the right temporal lobe, emphasizing the strong link between HSV encephalitis and seizures, which can be explained by various mechanisms. Timely antiviral therapy and tailored antiepileptic strategies led to gradual clinical improvement, illustrating the potential of valproate beyond its antiepileptic use. This case calls for further exploration into the pathophysiology and treatment of HSE, emphasizing individualized patient care and vigilance for potential post-resolution sequelae, contributing to our evolving understanding of the condition.
Keywords: Herpes Simplex Encephalitis; Ictal Vomiting; Diagnostic Challenges
HSV: Herpes Simplex Virus; HSV-1: Herpes simplex Virus Type 1; HSE: Herpes Simplex Encephalitis; CT: Computerized Tomography; CTA: Computerized Tomography Angiography; MRI: Magnetic Resonance Imaging; TB: Tuberculosis; EEG: Electroencephalogram; CSF: Cerebrospinal Fluid; PCR: Polymerase Chain Reaction; IL-6: Interleukin-6; TNF-α: Tumor Necrosis Factor-alpha; CNS: Central Nervous System; SE: Subsequent Epilepsy; ASS: Acute Symptomatic Seizures; PE: Postencephalitic Epilepsy; PLEDs: Periodic Lateralized Epileptiform Discharges; AEDs: Antiepileptic Drugs; AE: Autoimmune Encephalitis; GABA: Gamma-Aminobutyric Acid
Acute viral central nervous system (CNS) infections are a significant cause of acquired epilepsy, with Herpes Simplex Encephalitis (HSE) being the most common subtype, typically caused by Herpes Simplex Virus type 1 (HSV-1). HSV-1 reaches the brain by migrating from a primary oro-pharyngeal infection site via the trigeminal or olfactory nerves. This pathway explains the virus's preference for the medial temporal and inferior frontal lobes, regions associated with the olfactory pathway. By predominantly targeting the cerebral cortex, HSV-1 leads to an increased occurrence of seizures during HSE [1].
Focal seizures, followed by generalized seizures, constitute the primary seizure types associated with HSE, including alterations in autonomic functions [2]. This is particularly evident in temporal lobe epilepsies, where autonomic features, such as ictal vomiting, frequently serve as the initial and overt components of a seizure [3].
The case presented here involves HSE, marked by the onset of seizures during the acute phase that persist beyond resolution, with ictal vomiting emerging as the predominant symptom. The following sections delve into this case, providing a thorough examination and discussion of the clinical, radiological, and therapeutic aspects encountered while managing this unique manifestation of HSE.
A 60-year-old female with a medical history of diabetes, hypothyroidism, and hypertension presented to our hospital after experiencing three weeks of fever and recurrent vomiting. She had previously been hospitalized elsewhere for one week with similar symptoms but without improvement. Instead, her condition worsened, accompanied by behavioral changes and cognitive decline. Upon admission to our facility, she had abnormal jerky movements on the left side of her body lasting for minutes, followed by weakness.
Initial investigations, including a brain CT scan, revealed a hypodensity in the right fronto-temporal region [Figure 1], while CTA results were normal. Subsequent contrast-enhanced brain MRI showed an ill-defined abnormal signal predominantly affecting the cortical regions of the right temporal lobe, right hippocampus, posterior part of the right gyrus rectus, right insula, left hippocampus, and left fronto-parietal area. Patchy enhancement and mild linear lepto-meningeal enhancement were observed [Figure 2a, b], consistent with non-hemorrhagic herpetic encephalitis rather than an ischemic pattern.
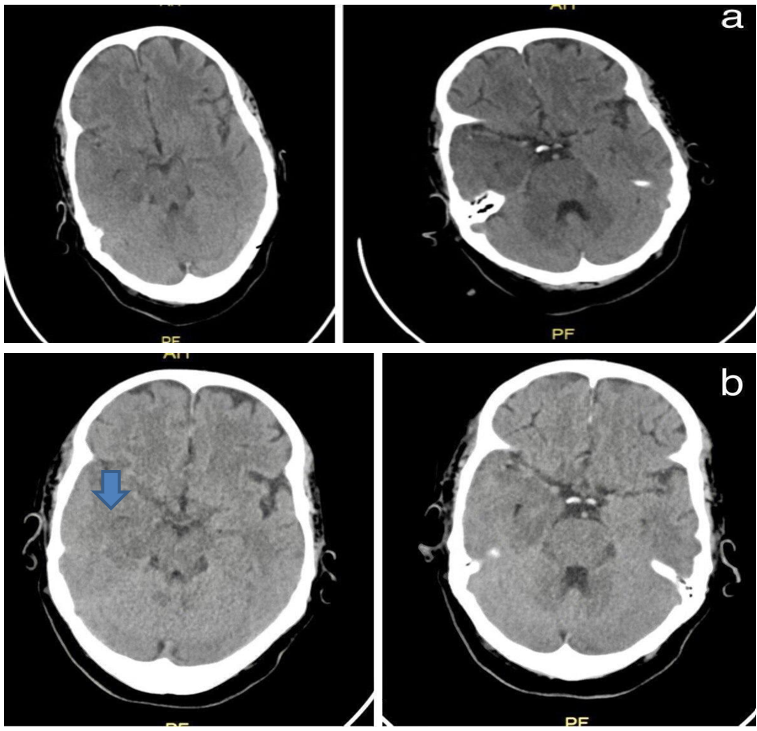
Figure 1: Axial Brain CT show hypoattenuation (darkness) of the brain parenchyma; loss of grey matter-white matter differentiation in the right fronto-temporal area. (a) Post admission day-1 (b) post admission day-2.
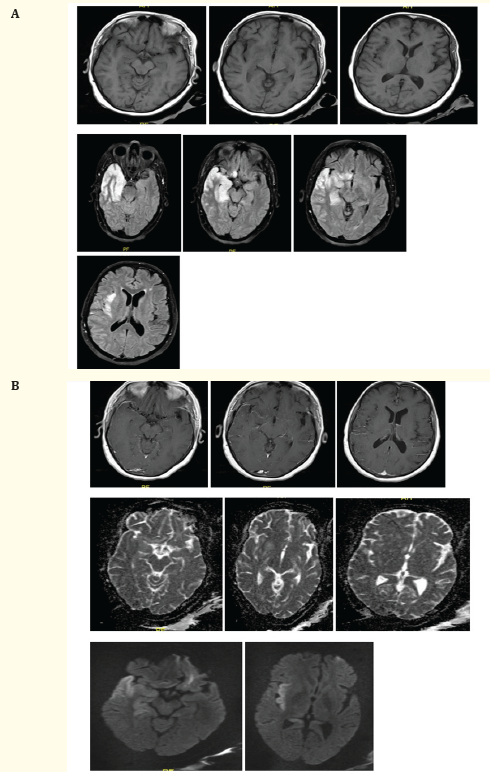
Figure 2A, B: Axial brain MRI show an ill-defined (predominantly cortical) area of abnormal signal intensity involving the right temporal lobe, right hippocampus, the posterior portion of right gyrus rectus and right insula, being hyperintense at both T2WI and FLAIR (blue arrow), hypo-intense at T1WI, with gyral swelling and diffusion restriction (prominent at insular region) (arrow head) with positive mass effect in the form of effacement of ipsilateral cortical sulci and sylvian fissure. Sparing of the right basal ganglia structures is seen. The left hippocampus also shows a subtle area of increased T2/FLAIR signal, with no diffusion restriction. Left fronto-parietal small white matter foci of abnormal signal being hyperintense at both T2 and FLAIR WI, isointense at T1WI, shows no diffusion restriction, no significant mass effect or peri-lesionsal edema. Post contrast study revealed subtle patchy enhancement of the involved region, associated with mild linear lepto-meningeal enhancement extending to the adjacent portions of the right parietal lobe
A lumbar puncture revealed significant pleocytosis with 500 leukocytes, predominantly lymphocytes (92%), a protein level of 106 mg/dL, normal glucose, and a positive HSV1 PCR result. CSF tests for T.B., Brucella, fungal culture, and gram stain were negative. EEG findings showed Periodic Lateralized Epileptiform Discharges (PLEDs) over the right hemisphere [Figure 3a]. The patient was diagnosed with HSE and started on IV acyclovir (10mg/kg/8 hours) and levetiracetam (500mg bid).
The patient gradually improved in left-sided weakness, behavioral changes, and cognitive impairment, with no further generalized seizures. However, vomiting persisted, initially decreasing in frequency for the first four days. After completing a 21-day course of IV acyclovir, she was discharged on antiemetics and levetiracetam. Two weeks later, she was readmitted due to persistent vomiting. Extensive investigations, including endoscopy, CT of the abdomen and pelvis, and laboratory tests, were negative. Repeat EEG showed no PLEDS but infrequent right temporal sharp waves [Figure 3b].
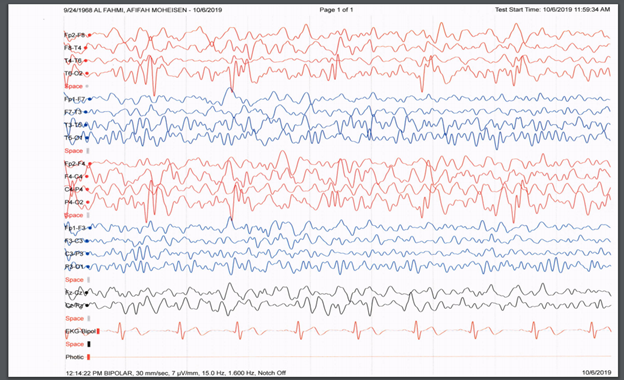
Figure 3A: EEG done at presentation showed PLEDS (blue arrows).
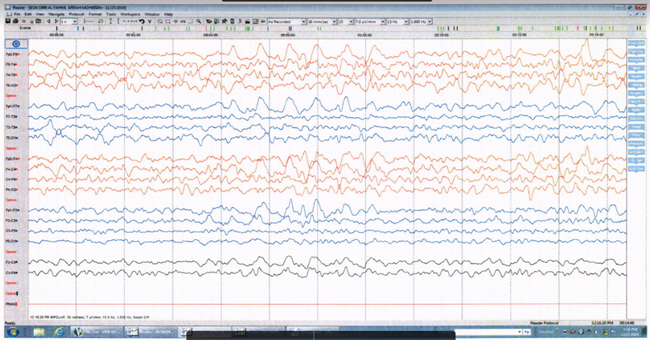
Figure 3b: EEG done 2 weeks later showed no more BLEDS but there is infrequent right temporal sharp waves (blue arrows).
Suspecting ictal vomiting as the cause of intractable vomiting, adjustments were made to the antiepileptic regimen, with the addition of valproate. This led to a remarkable response with a gradual decrease in vomiting frequency. Subsequent EEGs showed no epileptic activity, and follow-up examinations indicated a favourable outcome with the absence of vomiting and generalized seizures. While motor function improved, some residual cognitive impairment persisted during the three-month follow-up.
The patient's initial three-week history of fever and repeated vomiting presented diagnostic complexities, leading to an initial discharge without a definitive diagnosis. The subsequent emergence of abnormal jerky movements and weakness on admission highlighted the intricate nature of the case. Conclusive evidence for HSE was derived from neuroimaging results and CSF analysis, with EEG findings playing a crucial role in confirming the epileptic nature of the symptoms.
Studies indicate that seizures occur during the acute stage of Herpes Simplex Encephalitis (HSE) in approximately 75% of cases [4]. Unprovoked seizures are reported in 40–65% of instances, and Subsequent Epilepsy (SE) develops in 29%, often proving resistant to treatment [5]. A recent study with extensive follow-up on HSE patients found a significantly higher likelihood of developing post-encephalitis epilepsy (PE) in those who experience early seizures, particularly acute symptomatic seizures (ASS), with an eightfold increased risk. Younger age is identified as a risk factor, while findings from cerebrospinal fluid (CSF), imaging, and electroencephalogram (EEG) do not show a correlation with an elevated risk [6].
The mechanisms underlying epileptogenesis in Herpes Simplex Encephalitis (HSE) include neuronal injury, alterations in neural circuits, and the release of proinflammatory cytokines [7]. This process activates macrophages and microglia, leading to the production of interleukin (IL)-6 and tumor necrosis factor (TNF)-α [8]. IL-6 and TNF-α disrupt the balance between neuronal excitation and inhibition, increasing neuronal hyperexcitability [9]. Additionally, they may compromise the blood-brain barrier, causing cerebral edema, which can precipitate seizures [10].
Moreover, recent studies have indicated a possible link between central nervous system (CNS) viral infections and subsequent autoimmune encephalitis (AE) [11,12], which is another recognized cause of seizures and epilepsy. In a recent prospective study involving a group of patients with Herpes Simplex Encephalitis (HSE), 27% of cases developed AE characterized by autoimmune responses targeting N-methyl-D-aspartate receptor and other neuronal surface proteins within 3 months after antiviral therapy [13].
Interestingly, the focal seizures in our case primarily presented as ictal vomiting, a rare clinical manifestation believed to originate in the anterior part of the temporal lobe, specifically the amygdala or insula [14]. These regions are commonly affected in Herpes Simplex Encephalitis (HSE) [2], which aligns with the findings in our case. However, there is currently a lack of studies determining the frequency of ictal vomiting in HSE.
Ictal vomiting has been associated with the non-dominant hemisphere and is considered a lateralizing sign [14]. However, there are also reported cases where seizure onset has been lateralized to the dominant hemisphere [15].
In cases of ictal vomiting or retching, the interictal EEG can often appear normal, and excessive movement artifacts may hinder the identification of seizure onset [16]. However, in our case, EEG recordings revealed occasional right temporal sharp waves, which resolved following appropriate treatment.
When considering treatment, the influence of therapeutic approaches on the overall prognosis of patients with Herpes Simplex Encephalitis (HSE) is paramount. Initiating antiviral therapy promptly, such as with intravenous acyclovir, is crucial in managing HSE by suppressing viral replication and enhancing outcomes [17,18]. The efficacy of antiviral treatment is underscored by the patient's gradual recovery from left-sided weakness, behavioral changes, and cognitive impairment, as observed in our case.
Recent research has highlighted the significance of immune modulators as potential complementary treatments for Herpes Simplex Encephalitis (HSE). Immunotherapies, such as corticosteroids and intravenous immunoglobulins, have demonstrated effectiveness in regulating the inflammatory response linked to HSE, offering potential benefits in reducing long-term neurological complications [19,20].
Furthermore, identifying ictal vomiting as the underlying cause of refractory vomiting, supported by EEG findings, prompted the inclusion of valproate in the treatment plan. This addition resulted in a significant response with a gradual reduction in vomiting frequency, showcasing its potential beyond its antiepileptic effects. Valproate is known for its neuroprotective properties, anti-inflammatory actions, and modulation of gamma-aminobutyric acid (GABA)ergic neurotransmission, which may contribute to its efficacy in specific manifestations of HSE [21,22].
Incorporating diverse treatment modalities such as antiviral medications, immune modulators, and customized antiepileptic regimens reflects the dynamic evolution in managing Herpes Simplex Encephalitis (HSE). As we deepen our understanding of HSE pathophysiology, ongoing research and clinical trials hold promise in refining treatment approaches, thereby improving outcomes for patients affected by this condition.
Acknowledging the limitations of a single-case report, future research could explore the frequency of ictal vomiting in HSE cases and address any potential confounding factors affecting the generalizability of the findings.
In summary, this case of Herpes Simplex Encephalitis (HSE) underscores the diverse clinical spectrum and challenges in management. The patient's atypical presentation underscores the importance of considering HSE in patients with fever and unexplained persistent vomiting for early diagnosis and better prognosis.
Diagnostic tools (Neuroimaging, cerebrospinal fluid analysis, and electroencephalogram) confirmed HSE involvement in the right temporal lobe emphasizing the strong association between HSV encephalitis and seizures, which can be explained by various mechanisms.
Timely antiviral therapy and tailored antiepileptic strategies led to gradual clinical improvement, showcasing the potential of valproate beyond antiepileptic use.
This case prompts further exploration into HSE's pathophysiology and treatment. It emphasizes individualized patient care and vigilance for potential post-resolution sequelae, contributing to our evolving understanding of HSE.
Copyright: © 2024 Hosna Elshony., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.